911417-87-3
- Product Name:Belumosudil
- Molecular Formula:C26H24N6O2
- Purity:99%
- Molecular Weight:452.516
Product Details:
CasNo: 911417-87-3
Molecular Formula: C26H24N6O2
Factory Supply Belumosudil,Sale 911417-87-3 On Stock
- Molecular Formula:C26H24N6O2
- Molecular Weight:452.516
- Boiling Point:682.6±55.0 °C(Predicted)
- PKA:13?+-.0.40(Predicted)
- PSA:108.31000
- Density:1.318±0.06 g/cm3(Predicted)
- LogP:5.73340
Belumosudil(Cas 911417-87-3) Usage
|
Description |
Belumosudil is an oral inhibitor of Rho-associated coiled-coil-containing protein kinases (ROCK), specifically targeting ROCK2. It belongs to the class of drugs known as serine/threonine kinase inhibitors. By inhibiting ROCK2, Belumosudil modulates immune cell activity and prevents scar tissue buildup, which are key mechanisms involved in the pathogenesis of chronic GVHD. |
| Uses | Belumosudil is indicated for the treatment of chronic graft versus host disease (GVHD) in adults and children aged 12 years and older who have not responded to at least two other treatments. GVHD is a complication that can occur after hematopoietic stem-cell transplant (HSCT), where transplanted immune cells attack the recipient's tissues. Belumosudil helps to reduce immune cell activity and prevent tissue damage in individuals with chronic GVHD, thereby improving symptoms and quality of life. |
| Regulatory Approval | Ibrutinib was approved by regulatory agencies such as the FDA and the European Commission for the treatment of various blood cancers, including MCL and CLL/SLL. |
911417-87-3 Relevant articles
Safety, Tolerability, and Pharmacokinetics of Belumosudil, a Selective Rho-associated Coiled-coil-containing Protein Kinase 2 Inhibitor, in Healthy Japanese Volunteers: A PhaseⅠ, Randomized, Controlled Trial
Y Ogama,H Sato,A Miyata,K Kijima,Y Kumagai
, Japanese journal of clinical pharmacology, 2023
To assess the safety, tolerability, and pharmacokinetics of belumosudil, a selective Rho-associated coiled-coil-containing protein kinase 2 inhibitor, in healthy Japanese male adults, a phase Ⅰ, randomized, double-blind, placebo-controlled trial was conducted.Methods: In the single-dose study, 200, 400, or 800 mg of belumosudil or matching placebo was orally administered to 3 separate cohorts.
Budget Impact Analysis of Belumosudil for Chronic Graft-Versus-Host Disease Treatment in the United States
Carlos Bachier 1, Jeffrey Rolf Skaar 2, Sumudu Dehipawala 2, Benjamin Miao 3, Jonathan Ieyoub 4, Haya Taitel 4
Blood, Volume 138, Supplement 1, 23 November 2021, Page 1903
Belumosudil use was modeled in three different scenarios based on projections of branded agent shares (i.e., source of share from ibrutinib only, ruxolitinib only, or both ibrutinib and ruxolitinib in a market with branded agents only).
Belumosudil for Chronic Graft-Versus-Host Disease (cGVHD) after 2 or More Prior Lines of Therapy: The Rockstar Study (KD025-213)
Corey Cutler MD MPH, FRCPC 1, Stephanie J. Lee MDMPH 2, Sally Arai MD MS 3, Marcello Rotta MD 4, Behyar Zoghi MD * 5, Aravind Ramakrishnan MD 6, David Eiznhamer PhD * 7, Olivier Schueller PhD * 8, Zhongming Yang PhD * 9, Laurie S. Green MD * 10, Sanjay K. Aggarwal MD * 8, Bruce R. Blazar MD 11, Steven Z. Pavletic MD MS 12, Madan Jagasia MBBS, MS, MMHC 13
Blood,Volume 136, Supplement 1, 5 November 2020, Pages 45-46
This phase 2, open-label, randomized, multicenter study evaluated belumosudil 200 mg QD (n=66) and BID (n=66) in patients with cGVHD who received 2 to 5 prior lines of therapy (LOT). Treatment continued until clinically significant progression of cGVHD. The primary end point was overall response rate (ORR), defined per the 2014 National Institutes of Health Consensus Criteria.
911417-87-3 Process route
-
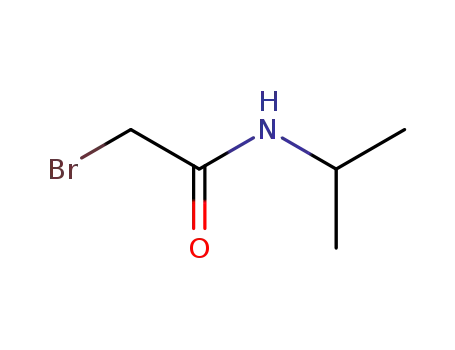
- 75726-96-4
2-bromo-N-isopropylacetamide

-
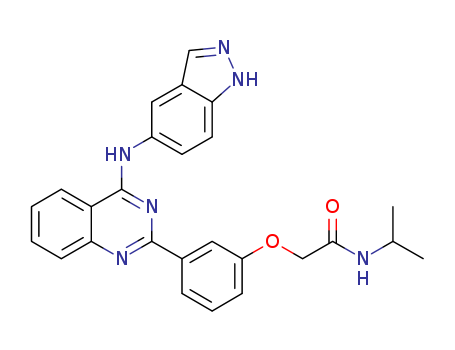
![2-[3-[4-(1H-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-N-propan-2-ylacetamide](/upload/2024/1/b5cf39f3-efca-4f71-9a4c-2b17fe038a72.png)
- 911417-87-3
2-[3-[4-(1H-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-N-propan-2-ylacetamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 7 steps
1: potassium carbonate / N,N-dimethyl-formamide / 2 h / 80 °C
2: sodium hydroxide / water; methanol; tetrahydrofuran / 20 °C
3: oxalyl dichloride / dichloromethane; N,N-dimethyl-formamide / 0.5 h / 20 °C / Inert atmosphere; Cooling with ice
4: N-ethyl-N,N-diisopropylamine / dichloromethane / 0.17 h / 20 °C / Cooling with ice; Inert atmosphere
5: potassium carbonate / water; ethanol / 2.5 h / 80 °C
6: thionyl chloride / N,N-dimethyl-formamide / 0.5 h / 20 °C / Inert atmosphere; Cooling with ice
7: N,N-dimethyl-formamide / 2.5 h / 100 °C / Sealed tube
With thionyl chloride; oxalyl dichloride; potassium carbonate; N-ethyl-N,N-diisopropylamine; sodium hydroxide; In tetrahydrofuran; methanol; ethanol; dichloromethane; water; N,N-dimethyl-formamide;
|
-
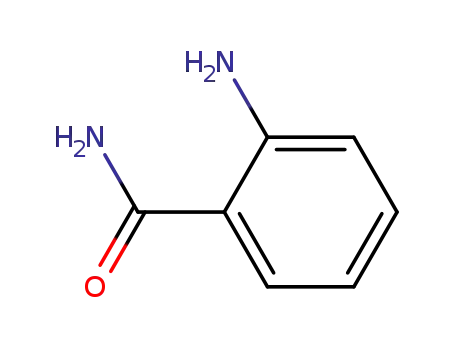
- 28144-70-9,88-68-6
anthranilic acid amide

-
![2-[3-[4-(1H-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-N-propan-2-ylacetamide](/upload/2024/1/b5cf39f3-efca-4f71-9a4c-2b17fe038a72.png)
- 911417-87-3
2-[3-[4-(1H-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-N-propan-2-ylacetamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 10 steps
1.1: pyridine / chloroform / 6 h / 20 °C
2.1: sodium hydroxide / water / 4 h / Heating / reflux
2.2: 2 h / 20 °C / pH 7
3.1: boron tribromide / dichloromethane / 4 h / -78 - 20 °C
3.2: 0.5 h / -78 - 20 °C
3.3: pH 7
4.1: pyridine / 3.5 h / 105 °C
5.1: thionyl chloride; N,N-dimethyl-formamide / 4 h / Heating / reflux
6.1: isopropyl alcohol / 0.5 h / 95 °C
7.1: methanol; ammonia; water / 48 h / 20 °C
8.1: potassium carbonate / N,N-dimethyl-formamide / 3.5 h / 80 °C
9.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
10.1: benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / dichloromethane; N,N-dimethyl-formamide / 0.25 h / 20 °C
10.2: 0.5 h / 20 °C
With pyridine; methanol; sodium hydroxide; thionyl chloride; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; ammonia; water; boron tribromide; potassium carbonate; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; In dichloromethane; chloroform; water; N,N-dimethyl-formamide; isopropyl alcohol;
|
911417-87-3 Upstream products
-
911417-62-4
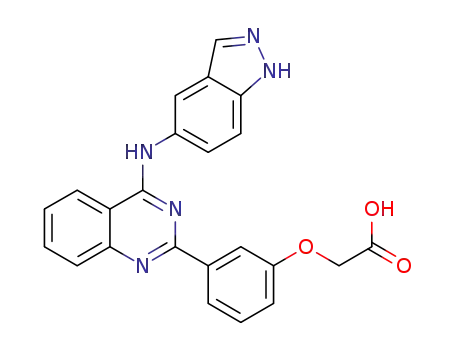
2-(3-(4-(1H-indazol-5-ylamino)quinazolin-2-yl)phenoxy)acetic acid
-
75-31-0
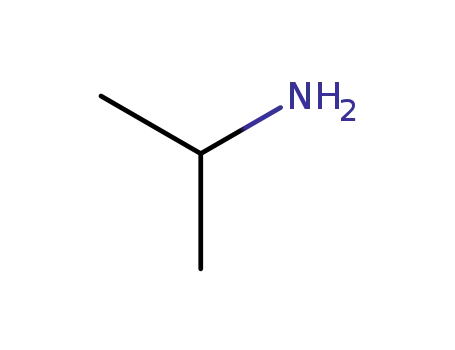
isopropylamine
-
19335-11-6
1H-indazol-5-ylamine
-
75726-96-4

2-bromo-N-isopropylacetamide
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Lasmiditan Hemisuccinate
CAS:439239-92-6
-
Opicapone
CAS:923287-50-7