923287-50-7
- Product Name:Opicapone
- Molecular Formula:C15H10Cl2N4O6
- Purity:99%
- Molecular Weight:413.174
Product Details:
CasNo: 923287-50-7
Molecular Formula: C15H10Cl2N4O6
Quality Manufacturer Supply Top Purity Opicapone 923287-50-7 Cheap Price
- Molecular Formula:C15H10Cl2N4O6
- Molecular Weight:413.174
- Boiling Point:701.1±70.0 °C (760 mmHg)
- PKA:4.67±0.38(Predicted)
- PSA:150.66000
- Density:1.80±0.1 g/cm3 (20 °C, 760 mmHg)
- LogP:4.59830
Opicapone(Cas 923287-50-7) Usage and Manufacturer
Opicapone is a medication used in conjunction with levodopa and carbidopa (commonly found in medications like Sinemet and Rytary) to address the end-of-dose "wearing-off" symptoms associated with Parkinson's disease. It belongs to the category of catechol-O-methyltransferase (COMT) inhibitors.
Parkinson's disease is a neurodegenerative disorder characterized by both motor and non-motor symptoms. Levodopa is considered the gold standard for symptom management in Parkinson's disease. However, after around two years of treatment, approximately half of patients experience fluctuations in motor performance. This is where COMT inhibitors like Opicapone become essential.
Opicapone, marketed under the brand name Ongentys, is a potent third-generation COMT inhibitor. Its mechanism of action involves inhibiting COMT, an enzyme responsible for metabolizing levodopa. By inhibiting COMT, Opicapone enhances the bioavailability and duration of action of levodopa, leading to more consistent symptom control throughout the day.
Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery.
923287-50-7 Relevant articles
Opicapone for the treatment of Parkinson’s disease: an update
András Salamon,Dénes Zádori,László Szpisjak,Péter Klivényi &László Vécsei
, Expert Opinion on Pharmacotherapy Volume 20, 2019 - Issue 18
This article summarizes our knowledge about a new third-generation COMT inhibitor, namely opicapone (OPC) (Search period: 2016–2019). The authors detail the pharmacological profile of OPC and summarize the results of completed clinical trials. In addition, they briefly summarize the achievements of the past few years.
Opicapone: A Review in Parkinson’s Disease
Adis Drug Evaluation
Drugs, Volume 76, pages 1293–1300, (2016)
No new unexpected safety concerns were identified after ≈1.4 years’ treatment with opicapone, with no serious cases of hepatotoxicity reported in clinical trials. With its convenient once-daily regimen, oral opicapone is an emerging COMT inhibitor option for use as adjunctive therapy to L-Dopa/DDCI therapy in adults with PD and end-of dose motor fluctuations who cannot be stabilized on those combinations.
Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial
Prof Joaquim J Ferreira, MD Prof Andrew Lees, MD José-Francisco Rocha, BSc Prof Werner Poewe, MD Prof Olivier Rascol, MD Prof Patrício Soares-da-Silva, MD
The Lancet Choice, VOLUME 15, ISSUE 2, P154-165, FEBRUARY 2016
Treatment with opicapone 50 mg was superior to placebo (mean difference in change from baseline −60·8 min, 95% CI −97·2 to −24·4; p=0·0015) and non-inferior to entacapone (−26·2 min, −63·8 to 11·4; p=0·0051). Treatment with opicapone 5 mg (p=0·056) or 25 mg (p=0·080) was not significantly different from treatment with placebo.
Effect of opicapone and entacapone on early morning-OFF pattern in Parkinson’s disease patients with motor fluctuations
A. Videnovic1 ∙ W. Poewe2 ∙ A. Lees3∙ … ∙ D. Magalhães7 ∙ J. Rocha6 ∙ P. Soares-da-Silva8
, Parkinsonism & Related Disorders, Volume 122106566May 2024
Opicapone (OPC), a once-daily catechol-O-methyltransferase inhibitor, was shown to be effective for end-of-dose motor fluctuations in Parkinson’s disease patients in two large multinational trials (BIPARK-I and II).
923287-50-7 Upstream products
-
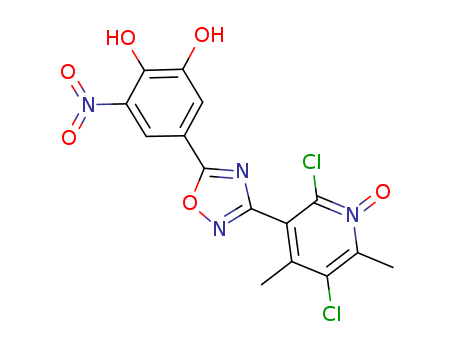
1044137-00-9
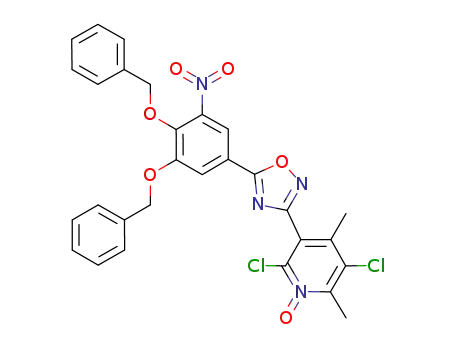
3-(5-(3,4-bis(benzyloxy)-5-nitrophenyl)-1,2,4-oxadiazol-3-yl)-2,5-dichloro-4,6-dimethylpyridine 1-oxide
-
123-54-6
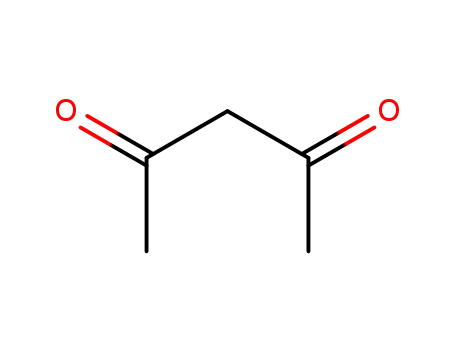
acetylacetone
-
769-28-8
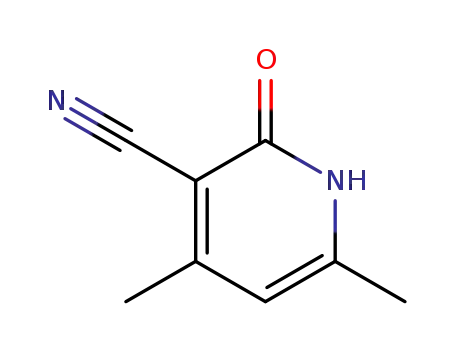
3-Cyano-4,6-dimethyl-2(1H)-pyridon
-
23819-92-3
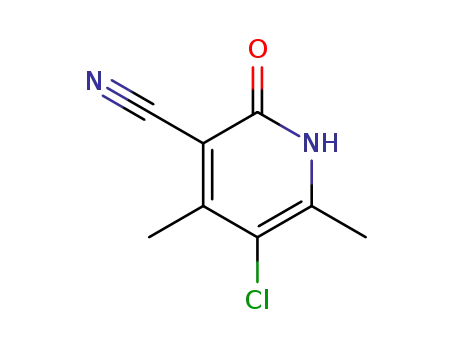
5-chloro-4,6-dimethyl-2-oxo-1,2-dihydropyridine-3-carbonitrile
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Belumosudil
CAS:911417-87-3
-
2'-Chloro acetophenone
CAS:2142-68-9