503612-47-3
- Product Name:Apixaban
- Molecular Formula:C25H25N5O4
- Purity:99%
- Molecular Weight:459.50
Product Details:
CasNo: 503612-47-3
Molecular Formula: C25H25N5O4
Appearance: white powder
Chinese Manufacturer Supply Top Purity 99% Apixaban 503612-47-3 with Efficient Delivery
- Molecular Formula:C25H25N5O4
- Molecular Weight:459.50
- Appearance/Colour:white powder
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.705
- Boiling Point:770.468 °C at 760 mmHg
- PKA:15.01±0.20(Predicted)
- Flash Point:419.764 °C
- PSA:110.76000
- Density:1.421 g/cm3
- LogP:3.52990
Apixaban(Cas 503612-47-3) Usage
|
Description |
Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. |
|
Type |
Apixaban is a factor Xa inhibitor, an anticoagulant. |
|
Notices |
It's important they know you're taking apixaban, as it may put you at risk of bleeding. You can drink alcohol while taking apixaban. But heavy drinking, especially binge drinking, can increase the effect of apixaban and make you more likely to bleed. Some side effects can be serious. If you experience any of these symptoms, call your doctor immediately or get emergency medical treatment: bleeding gums. nosebleeds. heavy vaginal bleeding. red, pink, or brown urine. red or black, tarry stools. coughing up or vomiting blood or material that looks like coffee grounds. |
| FDA approval | The US Food and Drug Administration (FDA) approved apixaban, also known by the brand name Eliquis, on December 28, 2012. |
| Chinese Manufacturer | Preparation of apixaban of formula (I), crystalline form a 0.5 g of apixaban crystalline form H2-2 is suspended in 25 ml acetone, and the dispersion is heated to the reflux temperature of the solvent. The dispersion is then cooled to room temperature. The solid is recovered by filtration through a Buckner funnel. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, At present, Huarong Pharm has successfully delivered innovative R&D products and services to more than 3,000 partners across the world. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
InChI:InChI=1/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32)
503612-47-3 Relevant articles
The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement
M.R. LASSEN 1, B.L. DAVIDSON 2, A. GALLUS 3, G. PINEO 4, J. ANSELL 5, D. DEITCHMAN 6
, Journal of Thrombosis and Haemostasis Volume 5, Issue 12, December 2007, Pages 2368-2375
All apixaban groups had lower primary efficacy event rates than either comparator. The primary outcome rate decreased with increasing apixaban dose (P = 0.09 with q.d./b.i.d. regimens combined, P = 0.19 for q.d. and P = 0.13 for b.i.d. dosing).
Apixaban, an oral direct factor Xa inhibitor, inhibits human clot-bound factor Xa activity in vitro
Xiaosui Jiang , Earl J. Crain , Joseph M. Luettgen , William A. Schumacher , Pancras C. Wong
, Thromb Haemost 2009; 101(04): 780-782
Apixaban in vitroobserved in this assay appears to be consistent with the observation in vivo that apixaban … Further, the IC50 concentrations of apixaban achieved in vitroin a proteinfree …
Relevant Products
-
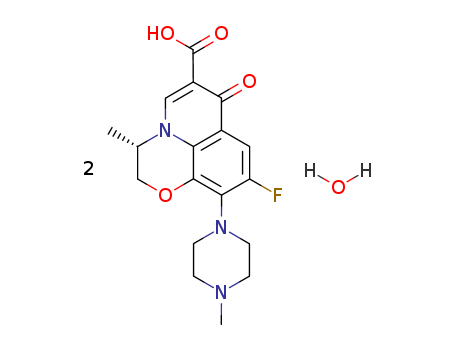
Pralsetinib
CAS:2097132-94-8
-
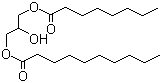
Decanoyl/octanoyl-glycerides
CAS:65381-09-1
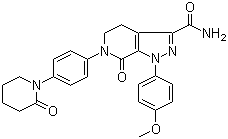
-
Levofloxacin Hemihydrate
CAS:138199-71-0