2097132-94-8
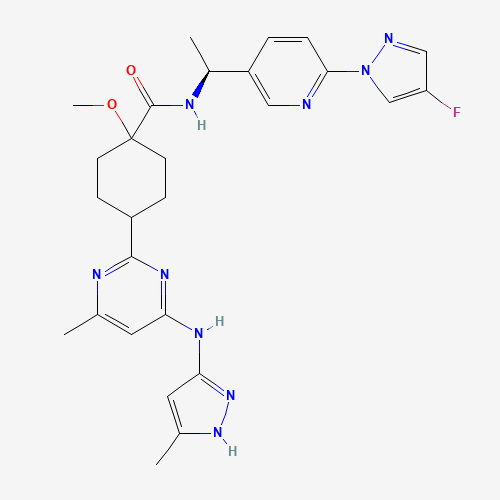
- Product Name:Pralsetinib
- Molecular Formula:C27H32FN9O2
- Purity:99%
- Molecular Weight:533.6
Product Details:
CasNo: 2097132-94-8
Molecular Formula: C27H32FN9O2
Quality Factory Supply Top Purity 99% Pralsetinib 2097132-94-8 Safe Transportation
- Molecular Formula:C27H32FN9O2
- Molecular Weight:533.6
Pralsetinib(Cas 2097132-94-8) Usage
|
Description |
Pralsetinib is a tyrosine kinase inhibitor, available in oral form, that selectively targets and inhibits specific proteins involved in cancer cell growth and proliferation. It is designed to block the activity of the RET (rearranged during transfection) protein, which is mutated or fused in certain types of cancer cells. By inhibiting RET signaling, pralsetinib helps to suppress tumor growth and progression. |
| Uses |
Pralsetinib is indicated for the treatment of the following conditions: Metastatic non-small cell lung cancer (NSCLC): Pralsetinib is used to treat adults and children aged 12 years and older with metastatic NSCLC that is positive for RET fusion as detected by an FDA-approved test. Thyroid cancer: Pralsetinib is indicated for the treatment of adults and children aged 12 years and older with refractory thyroid cancer that is positive for RET mutation or fusion. This includes medullary thyroid cancer (MTC) and differentiated thyroid cancer (DTC) that is refractory to radioactive iodine therapy. |
2097132-94-8 Relevant articles
Green One-Pot Chemo-Enzymatic Synthesis of a Key Chiral Amine Intermediate:Useful to Pralsetinib Synthesis
G Rajendra,K Ganesh,RG Govinda
, Chemistry Select, 2023
This study hence represents a mild and scalable procedure for the synthesis of the chiral intermediate of Pralsetinib with yields exceeding 80%,which forms a crucial step in the synthesis of the anti-cancer drug.
Clinical activity and safety of the RET inhibitor pralsetinib in patients with RET fusion-positive solid tumors: Update from the ARROW trial.
V Subbiah,PA Cassier,S Siena,G Alonso,LG Paz-Ares,P Garrido,E Nadal,G Curigliano,J Vuky,G Lopes
Journal of Clinical Oncology, 2021
Here we provide an update on the clinical activity of pralsetinib in patients (pts) with advanced RET fusion-positive solid tumors other than NSCLC and thyroid cancer ("other" RET fusion–positive solid tumors).
Relevant Products
-
Levofloxacin Hemihydrate
CAS:138199-71-0
-
Apixaban
CAS:503612-47-3
-
Oxaliplatin
CAS:61825-94-3