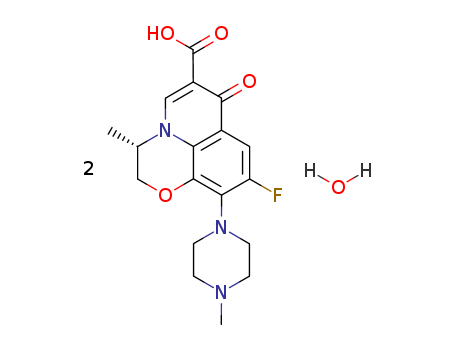
138199-71-0
- Product Name:Levofloxacin Hemihydrate
- Molecular Formula:2(C18H20FN3O4).H2O
- Purity:99%
- Molecular Weight:740.761
Product Details:
CasNo: 138199-71-0
Molecular Formula: 2(C18H20FN3O4).H2O
Appearance: pale yellow solid
Buy Quality Levofloxacin Hemihydrate,Hot Sale 138199-71-0 In Stock
- Molecular Formula:2C18H20FN3O4*H2O
- Molecular Weight:740.761
- Appearance/Colour:pale yellow solid
- Vapor Pressure:6.7E-14mmHg at 25°C
- Melting Point:214-216 °C
- Boiling Point:571.5 °C at 760 mmHg
- Flash Point:299.4 °C
- PSA:84.24000
- Density:1.48 g/cm3
- LogP:1.48260
Levofloxacin Hemihydrate(Cas 138199-71-0) Usage
|
Description |
Levofloxacin Hemihydrate is a hydrated form of levofloxacin, a synthetic antibiotic from the fluoroquinolone class. It is used primarily for the treatment of bacterial infections affecting different parts of the body, and its hemihydrate formulation ensures stability under ambient conditions. |
|
Uses |
Levofloxacin Hemihydrate is classified as a broad-spectrum fluoroquinolone antibiotic. It is used to treat various bacterial infections and is particularly effective against both Gram-positive and Gram-negative bacteria. Levofloxacin hemihydrate works by inhibiting bacterial DNA gyrase and topoisomerase IV, enzymes essential for DNA replication, transcription, repair, and recombination. This inhibition ultimately leads to bacterial cell death, making it highly effective against a wide range of bacterial pathogens. Levofloxacin hemihydrate is used to treat bacterial infections in the respiratory tract, such as pneumonia (community-acquired and nosocomial) and acute bacterial sinusitis. It is also prescribed for treating acute pyelonephritis and complicated and uncomplicated UTIs. |
| Pharmacokinetics | Absorption: Levofloxacin is well absorbed when taken orally, with a bioavailability of approximately 99%. Food does not significantly affect its absorption. Distribution: It has excellent tissue penetration, with concentrations higher in tissues than in plasma. Metabolism: Levofloxacin undergoes limited metabolism in the liver. Excretion: Primarily excreted unchanged in the urine. |
InChI:InChI=1/2C18H20FN3O4.H2O/c2*1-10-9-26-17-14-11(16(23)12(18(24)25)8-22(10)14)7-13(19)15(17)21-5-3-20(2)4-6-21;/h2*7-8,10H,3-6,9H2,1-2H3,(H,24,25);1H2/t2*10-;/m00./s1
138199-71-0 Relevant articles
PROCESS FOR OBTAINING LEVOFLOXACIN FREE FROM SALTS
-
Page/Page column 7, (2008/06/13)
This invention relates to a process for ...
Understanding the Dehydration of Levofloxacin Hemihydrate
Eric M. Gorman, Brian Samas, Eric J. Munson
, Journal of Pharmaceutical Sciences, Volume101, Issue9 Special Issue:Dedicated to Professor Valentino Stella September 2012 Pages 3319-3330
In this study, differential scanning calorimetry (DSC), thermogravimetric analysis, Raman spectroscopy, single-crystal and powder X-ray diffraction, and solid-state NMR spectroscopy were used to investigate the transitions that occurred upon dehydration to the anhydrate as well as additional transitions that occurred to the anhydrous material upon heating/cooling.
Fabrication and evaluation of levofloxacin hemihydrate floating tablet
VT Thakkar, PA Shah, TG Soni, MY Parmar, MC Gohel, TR Gandhi
, Research in Pharmaceutical Sciences
The release rate of levofloxacin hemihydrates from matrices was mainly controlled by the hydrophilic and hydrophobic polymer ratio. Matrix tablet containing 25% HPMC K4M and 15% Gelucire 43/01 (F4 batch) showed a release as target profile. Optimal batch (F4) was selected by regression analysis which followed Higuchi kinetic. Novel mathematical approach was applied to determine the deviation in area under the curve (AUC) between predicated and observed dissolution data which found to be lowest in optimal batch.
138199-71-0 Upstream products
-
177472-30-9
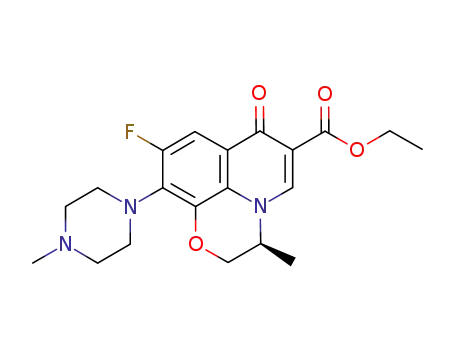
C20H24FN3O4
-
109-01-3
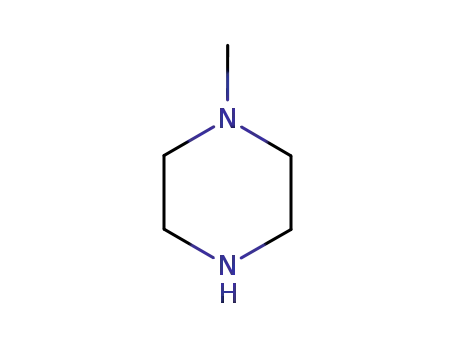
1-methyl-piperazine
-
100986-89-8
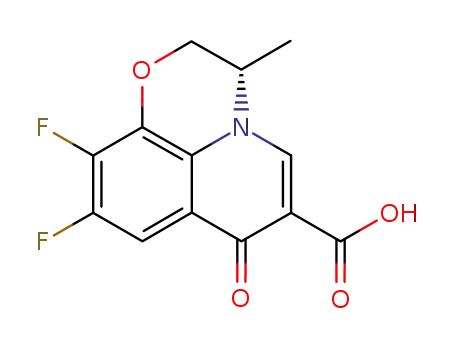
levofloxacin Q-acid
Relevant Products
-
Heparin Sodium
CAS:9041-08-1
-
Pralsetinib
CAS:2097132-94-8
-
Oxaliplatin
CAS:61825-94-3