9041-08-1
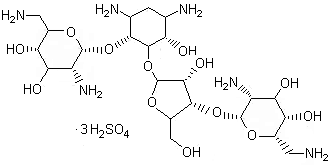
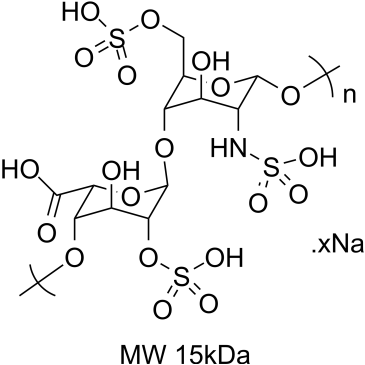
- Product Name:Heparin Sodium
- Molecular Formula:(C12H16NS2Na3)20
- Purity:99%
- Molecular Weight:
Product Details:
CasNo: 9041-08-1
Molecular Formula: (C12H16NS2Na3)20
Buy High Quality Heparin Sodium ,99% Pure 9041-08-1 Safe Transportation
- Molecular Formula:Unspecified
- Molecular Weight:
- PSA:0.00000
- LogP:0.00000
Heparin Sodium(Cas 9041-08-1) Usage
|
Description |
Heparin Sodium, USP is a heterogenous group of straight-chain anionic mucopolysaccharides, called glycosamino-glycans having anticoagulant properties. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. |
|
Uses |
HEPARIN SODIUM INJECTION is an anticoagulant indicated for: Prophylaxis and treatment of venous thrombosis and pulmonary embolism Prophylaxis and treatment of the thromboembolic complications associated with atrial fibrillation Treatment of acute and chronic consumption coagulopathies Prevention of clotting in arterial and cardiac surgery Prophylaxis and treatment of peripheral arterial embolism Anticoagulant use in transfusion, extracorporeal circulation, and dialysis procedures |
|
Others |
Heparin sodium may prolong one-stage prothrombin time; when heparin sodium is given with dicumarol or warfarin sodium, a period of at least 5 hr after last intravenous dose or 24 hr after last subcutaneous dose should elapse before blood is drawn if a valid prothrombin time is to be obtained. |
9041-08-1 Relevant articles
Acylated non-α-amino acids as novel agents for the oral delivery of heparin sodium, USP
Andrea Leone-Bay, Duncan R Paton, Bruce Variano, Harry Leipold, Theresa Rivera, Judy Miura-Fraboni, Robert A Baughman, Noemi Santiago
Journal of Controlled Release Volume 50, Issues 1–3, 2 January 1998, Pages 41-49
Heparin activity is typically measured by an increase in plasma clotting time using the prothrombin time (PT) and/or the activated partial thromboplastin time (APTT) assays. The therapeutic target range is 1.5–2.5 times baseline [2], which can be obtained easily by titrating the dose and measuring APTT.
Warfarin Sodium versus Low-Dose Heparin in the Long-Term Treatment of Venous Thrombosis
List of authors. Russell Hull, M.B., B.S., Terry Delmore, M.SC, Edward Genton, M.D., Jack Hirsh, M.D., Michael Gent, M.SC, David Sackett, M.D., M.SC., Dermot McLoughlin, M.B., B.S., and Peter Armstrong, M.D.
, N Engl J Med 1979; 301:855-858
Seven patients on warfarin sodium experienced bleeding complications (of which four were major), as compared with no patients receiving subcutaneous heparin (P<0.005). Thus, adjusted-dose warfarin sodium is more effective than low-dose subcutaneous heparin in preventing recurrent venous thromboembolism, but its use is accompanied by a significant risk of bleeding.
9041-08-1 Downstream products
-
9005-49-6
heparin
Relevant Products
-
Neomycin Sulphate
CAS:1405-10-3
-
Danuglipron
CAS:2230198-02-2
-
Tirofiban Hcl
CAS:142373-60-2