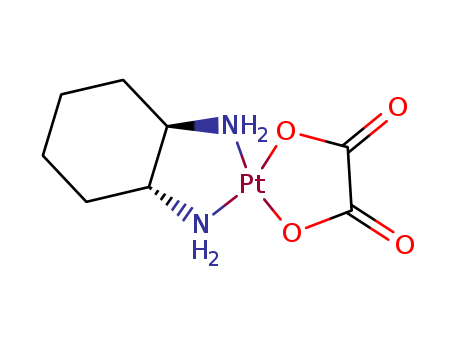
61825-94-3
- Product Name:Oxaliplatin
- Molecular Formula:C8H14N2O4Pt
- Purity:99%
- Molecular Weight:397.29
Product Details:
CasNo: 61825-94-3
Molecular Formula: C8H14N2O4Pt
Appearance: White crystalline solid
Quality Manufacturer Supply Best Quality Oxaliplatin 61825-94-3 Efficient Transportation
- Molecular Formula:C8H14N2O4Pt
- Molecular Weight:397.29
- Appearance/Colour:White crystalline solid
- Boiling Point:193.6oC at 760 mmHg
- Flash Point:75oC
- PSA:104.64000
- LogP:0.61450
Oxaliplatin(Cas 61825-94-3) Usage
|
Description |
Oxaliplatin is a platinum-based chemotherapeutic agent used primarily to treat metastatic colorectal cancer. It belongs to a class of drugs known as DNA-crosslinking agents, which inhibit DNA synthesis in cancer cells by forming DNA-DNA and DNA-protein crosslinks, leading to cell death (apoptosis). It is commonly used in combination with other chemotherapy drugs. |
| Mechanism of Action | Oxaliplatin forms intrastrand and interstrand crosslinks within DNA, preventing DNA replication and transcription. This leads to cellular apoptosis, particularly in rapidly dividing cancer cells. |
|
Originator |
Bebiopharm (Switzerland) |
|
Uses |
Oxaliplatin is primarily used in the treatment of metastatic colorectal cancer. It is often part of combination chemotherapy regimens like FOLFOX (5-fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin). Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
|
Brand name |
Eloxatin (Sanofi Aventis). |
| Administration | Oxaliplatin is administered intravenously, typically in combination with other chemotherapy agents. The dosage and treatment cycle depend on factors such as the patient's overall health, cancer stage, and other treatment considerations. Commonly, it is given once every two weeks as part of the FOLFOX regimen. |
InChI:InChI=1/C6H14N2.C2H2O4.Pt/c7-5-3-1-2-4-6(5)8;3-1(4)2(5)6;/h5-6H,1-4,7-8H2;(H,3,4)(H,5,6);/q;;+2/p-2/t5-,6-;;/m1../s1/rC8H14N2O4Pt/c11-7-8(12)14-15(13-7)9-5-3-1-2-4-6(5)10-15/h5-6H,1-4,9-10H2/t5-,6-/m1/s1
61825-94-3 Relevant articles
Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin
Robert A. Nagourney a , Steven Evans a , Peter H. Tran a , Adam J. Nagourney a , Paul H. Sugarbaker b
, European Journal of Surgical Oncology Volume 47, Issue 4 , April 2021, Pages 738-742
Of 87 fresh colon cancer specimens, 54 (62%) were untreated and 33 (38%) had received prior folinic acid, 5-fluorouracil, oxaliplatin (FOLFOX) or capecitabine and oxaliplatin (CAPOX). The failure of PRODIGE 7 to improve survival with surgery plus HIPEC following NACT may reflect diminished oxaliplatin cytotoxicity in patients whose residual disease has been selected for oxaliplatin and 5-FU resistance.
Cellular and Molecular Pharmacology of Oxaliplatin
Eric Raymond; Sandrine Faivre; Stephen Chaney; Jan Woynarowski; Esteban Cvitkovic
, Mol Cancer Ther (2002) 1 (3): 227–235.
In in vivo studies, oxaliplatin is active against breast, colon, and gastric cancer; renal cell carcinoma; and sarcoma (16). In addition, oxaliplatin has been tested in vitro and in vivo against cisplatin-resistant cell lines and tumor models, including human ovarian, lung, cervix, colon, and leukemia cell lines.
Targeting strategies for oxaliplatin-induced peripheral neuropathy: clinical syndrome, molecular basis, and drug development
Yang Yang, Bing Zhao, Xuejiao Gao, Jinbing Sun, Juan Ye, Jun Li & Peng Cao
Journal of Experimental & Clinical Cancer Research, Volume 40, article number 331, (2021)
Oxaliplatin (OHP)-induced peripheral neurotoxicity (OIPN) is a severe clinical problem and potentially permanent side effect of cancer treatment. For the management of OIPN, accurate diagnosis and understanding of significant risk factors including genetic vulnerability are essential to improve knowledge regarding the prevalence and incidence of OIPN as well as enhance strategies for the prevention and treatment of OIPN.
61825-94-3 Upstream products
-
61848-66-6
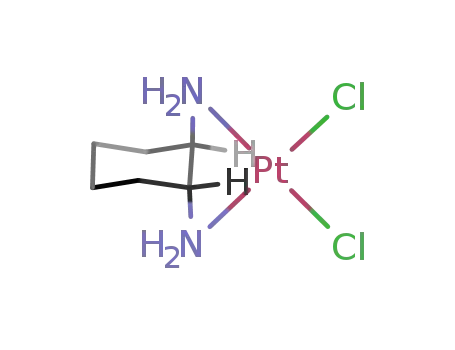
(1R,2R)-1,2-diaminocyclohexanedichloroplatinum(II)
-
33081-83-3
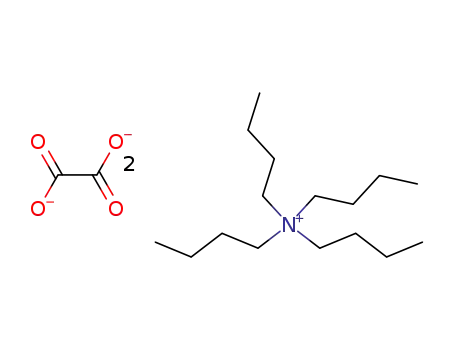
bis(tetrabutylammonium)oxalate
-
144-62-7
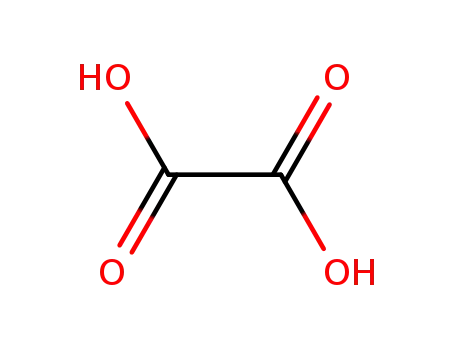
oxalic acid
-
583-52-8
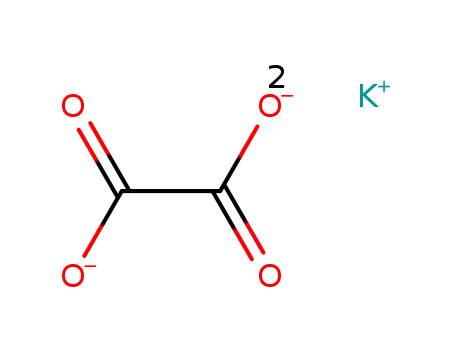
potassium oxalate
61825-94-3 Downstream products
-
111263-58-2
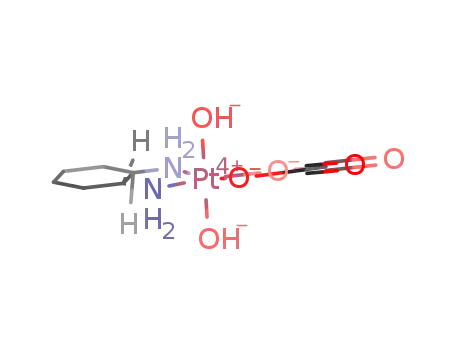
cis-oxalic acid (trans-1,2-cyclohexanediamine)dihydroxyplatinum(II)
-
2864-70-2
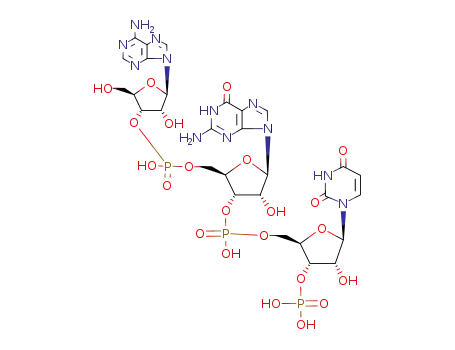
5'-AGU-3'
Relevant Products
-
Nedaplatin
CAS:95734-82-0
-
Levofloxacin Hemihydrate
CAS:138199-71-0
-
Sparsentan
CAS:254740-64-2