254740-64-2
- Product Name:Sparsentan
- Molecular Formula:C32H40N4O5S
- Purity:99%
- Molecular Weight:592.75
Product Details:
CasNo: 254740-64-2
Molecular Formula: C32H40N4O5S
Buy High Quality Sparsentan 254740-64-2 Efficient Shipping
- Molecular Formula:C32H40N4O5S
- Molecular Weight:592.75
- Melting Point:148 °C(Solv: isopropanol (67-63-0); water (7732-18-5))
- Boiling Point:744.4±70.0 °C(Predicted)
- PKA:7.06±0.50(Predicted)
- PSA:122.48000
- Density:1.28±0.1 g/cm3(Predicted)
- LogP:7.06670
Sparsentan (Cas 254740-64-2) Usage
|
Description |
Sparsentan is a first-in-class medication that acts as a dual-acting angiotensin receptor blocker (ARB) and highly selective endothelin Type A receptor antagonist. This unique mechanism of action allows sparsentan to target multiple pathways involved in kidney disease, making it potentially more effective than traditional treatments that target only one pathway. |
| Uses |
Sparsentan is indicated for the treatment of two specific kidney conditions: Primary Immunoglobulin A Nephropathy (IgAN): IgAN is a kidney disease characterized by inflammation and damage to the glomeruli, the filtering units of the kidneys. Sparsentan helps to reduce protein levels in the urine and may slow the progression of kidney damage in patients with IgAN. Focal Segmental Glomerulosclerosis (FSGS): FSGS is another type of kidney disease that affects the glomeruli. Sparsentan is being investigated as a potential treatment for FSGS in clinical trials. It is currently in Phase 3 development for this indication. |
254740-64-2 Relevant articles
Efficacy and Safety of Sparsentan Compared With Irbesartan in Patients With Primary Focal Segmental Glomerulosclerosis: Randomized, Controlled Trial Design (DUET)
R Komers,DS Gipson,P Nelson,S Adler,T Srivastava,VK Derebail,KE Meyers,P Pergola,ME Macnally,JL Hunt
Kidney International Reports, 2017
Patients aged 8 to 75 years with primary FSGS will be randomized to treatment with sparsentan or irbesartan for 8 weeks. The primary efficacy objective is to test the hypothesis that sparsentan over the dose range (200 mg, 400 mg, or 800 mg daily) is superior to irbesartan...
USE OF SPARSENTAN FOR THE TREATMENT OF CHRONIC INFLAMMATORY DISEASES
Zhang,Jinkun,Dziewanowska,E Zofia,Belder,Rene,Henderson,Ian,Bogardus,B Joseph
EP3708163A1
Methods of administering and pharmaceutical compositions of a biphenyl sulphonamide compound which is a dual angiotensin and endothelin receptor antagonist are disclosed for treating diseases.
Dual angiotensin II and endothelin A receptor antagonists: Synthesis of 2′-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics
Murugesan, Natesan,Gu, Zhengxiang,Fadnis, Leena,Tellew, John E.,Baska, Rose Ann F.,Yang, Yifan,Beyer, Sophie M.,Monshizadegan, Hossain,Dickinson, Kenneth E.,Valentine, Maria T.,Humphreys, W. Griffith,Lan, Shih-Jung,Ewing, William R.,Carlson, Kenneth E.,Kowala, Mark C.,Zahler, Robert,Macor, John E.
, p. 171 - 179 (2007/10/03)
In a previous report we demonstrated tha...
254740-64-2 Process route
-
![4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl-N-](4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-N-(methoxymethyl) [1,1'-biphenyl]-2-sulfonamide](/upload/2024/1/abb9bbe2-925b-4654-b144-89a4c36c4aea.png)
- 1026355-73-6
4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl-N-](4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-N-(methoxymethyl) [1,1'-biphenyl]-2-sulfonamide

-
![4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl) [1,1'-biphenyl]-2-sulfonamide](/upload/2024/1/f6349b39-b7b7-46a7-a1e5-f6809159640d.png)
- 254740-64-2
4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl) [1,1'-biphenyl]-2-sulfonamide
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In ethanol; water; for 1h; Reflux;
|
88% |
|
With hydrogenchloride; In ethanol; for 2h; Heating;
|
1.0 g |
|
4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl-N-](4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-N-(methoxymethyl) [1,1'-biphenyl]-2-sulfonamide; With hydrogenchloride; In ethanol; water; at 75 - 80 ℃; for 2h;
With sodium hydroxide; In ethanol; water; at 20 ℃; pH=8;
|
-
-
C36H48N4O7S

-
![4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl) [1,1'-biphenyl]-2-sulfonamide](/upload/2024/1/f6349b39-b7b7-46a7-a1e5-f6809159640d.png)
- 254740-64-2
4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl]-N-(4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl) [1,1'-biphenyl]-2-sulfonamide
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In ethanol; water; for 1h; Reflux;
|
86% |
254740-64-2 Upstream products
-
1026355-73-6
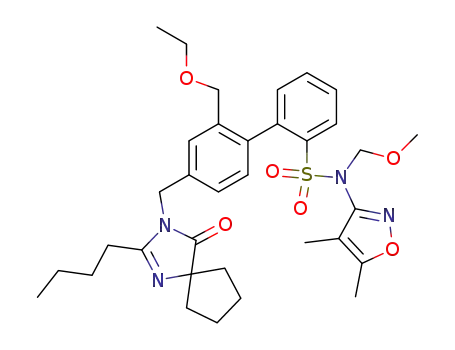
4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]non-1-en-3-yl)methyl-N-](4,5-dimethyl-3-isoxazolyl)-2'-(ethoxymethyl)-N-(methoxymethyl) [1,1'-biphenyl]-2-sulfonamide
-
41963-20-6
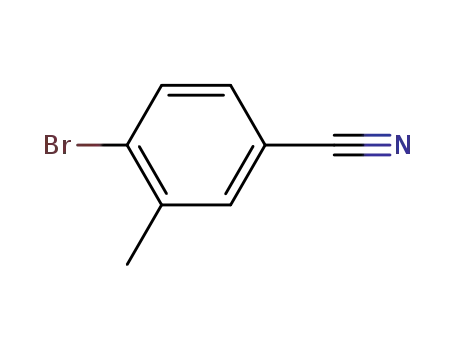
4-bromo-3-methylbenzonirtile
-
190197-86-5
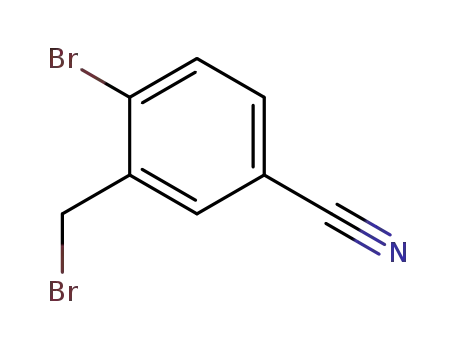
4-bromo-3-(bromomethyl)benzonitrile
-
837408-71-6
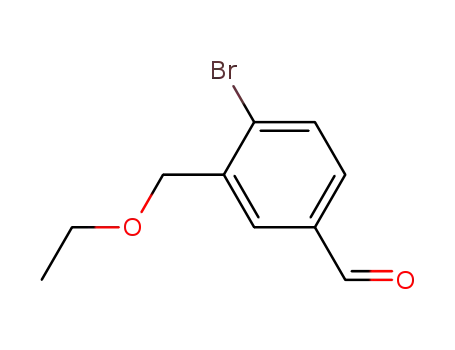
4-bromo-3-(ethoxymethyl)benzaldehyde
Relevant Products
-
Tinidazole
CAS:19387-91-8
-
Oxaliplatin
CAS:61825-94-3
-
Milvexian
CAS:1802425-99-5