439239-92-6
- Product Name:Lasmiditan Hemisuccinate
- Molecular Formula:C42H42F6N6O8
- Purity:99%
- Molecular Weight:436.411
Product Details:
CasNo: 439239-92-6
Molecular Formula: C42H42F6N6O8
Buy High Quality Lasmiditan Hemisuccinate ,Reliable Quality 439239-92-6 Customized Supply
- Molecular Formula:0C4H6O4*C19H18F3N3O2
- Molecular Weight:436.411
- PSA:206.18000
- LogP:7.13100
Lasmiditan Hemisuccinate(Cas 439239-92-6) Usage
|
Description |
Lasmiditan hemisuccinate is a white, crystalline powder which is sparingly soluble in water, slightly soluble in ethanol, and soluble in methanol. Lasmiditan Hemisuccinate is an oral medication primarily utilized for the acute treatment of migraine headaches in adults. Marketed under the brand name Reyvow, it is available as a white, crystalline powder with limited solubility in water, slight solubility in ethanol, and solubility in methanol. Lasmiditan acts as a highly selective serotonin 5-HT(1F) receptor agonist. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
| Function | Migraine headaches are characterized by severe throbbing pain often accompanied by symptoms like nausea, sensitivity to sound and light, and in some cases, aura. Lasmiditan works by binding to and activating 5-HT(1F) receptors, leading to the constriction of blood vessels and the inhibition of certain pain pathways associated with migraine attacks. |
| Uses | Approved by the U.S. Food and Drug Administration (FDA) in October 2019, Lasmiditan represents a significant advancement in migraine treatment due to its unique mechanism of action as a serotonin receptor agonist. It became available in February 2020 in the United States and received approval from the European Commission on August 17, 2022, further expanding its availability for migraine sufferers. Developed by Eli Lilly, Lasmiditan Hemisuccinate is considered a first-in-class medication for its novel approach in targeting migraine symptoms. |
439239-92-6 Relevant articles
A Reverse phase HPLC method Development and Validation of 2,4,6 Trifluro Benzoic acid and its impurities: A Key Raw Material Used In Preparation of Anti Migraine Drug Lasmiditan Hemisuccinate
M. Maheswar Reddy; A. Raghavendra; B. Kishore; E. Venkat Reddy; D. Yakaiah; V.B. Surya Varma; T. Karthikeyan; A.N. Kishore Kumar Reddy; P. Vijay Bhaskar
, Egyptian Journal of Chemistry, Volume 66, Issue 10, October 2023, Page 49-53
The impurities formed during the synthesis of TBA will diminishes the pharmacological quality of the Lasmiditan Hemisuccinate. Hence, the stringent control of TBA and its related impurities were significantly important to achieve the niche quality of Lasmiditan Hemisuccinate. This HPLC method developed by using Zorbax SB-Aq, 5 µm, 4.6 x 250 mm column with mobile phase containing a gradient mixture of solvent A and B. The buffer is 0.1% triethyl amine solution is solvent A and acetonitrile, methanol, water in the ratio of 700:200:100 v/v/v solvent mixture is solvent B.
Lasmiditan Hemisuccinate: Selective Separation and Validation of Process Related Impurities by HPLC
P. Vijay Bhaskar, E. Venkata Reddy, M. Maheswar Reddy, A. Raghavendra
Paragraph 0094, (2021/08/06)
A rapid and efficient gradient RP-HPLC method was developed for the selective separation and determination of process related impurities of Lasmiditan Hemisuccinate (LDT) and its degradation products in bulk drugs. Chromatographic separation of five process related impurities of LDT was tested on Eclipse XDB C8, Primasil C18, Inertsil ODS 3V, and Zorbax SB-phenyl columns for efficient separation.
PROCESSES AND INTERMEDIATE FOR THE LARGE-SCALE PREPARATION OF 2,4,6-TRIFLUORO-N-[6-(1-METHYL-PIPERIDINE-4-CARBONYL)-PYRIDIN-2-YL]-BENZAMIDE HEMISUCCINATE, AND PREPARATION OF 2,4,6-TRIFLUORO-N-[6-(1-METHYL-PIPERIDINE-4-CARBONYL)-PYRIDIN-2-YL]-BENZAMIDE ACE
-
Page/Page column 37-40, (2021/01/29)
The embodiments of present invention pro...
439239-92-6 Upstream products
-
439239-90-4
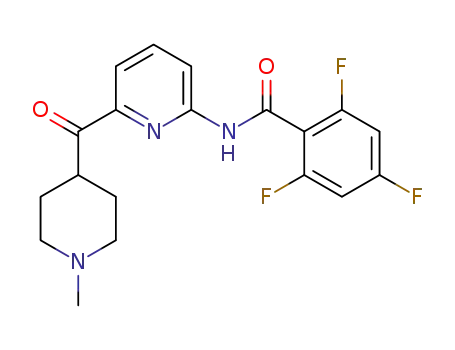
Lasmiditan
-
110-15-6
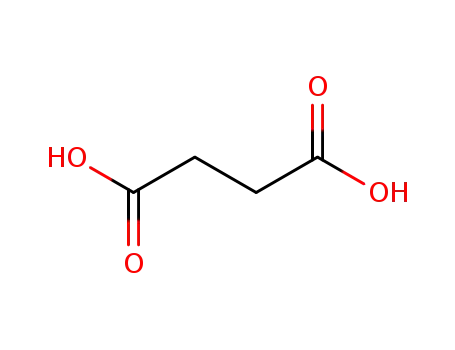
succinic acid
-
5570-77-4
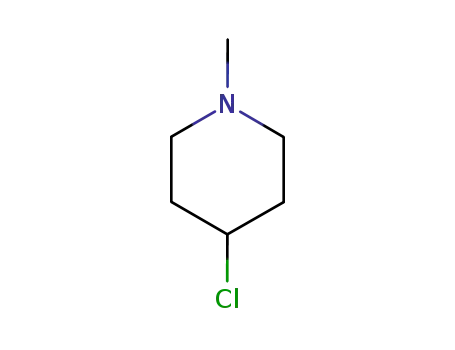
4-chloro-1-methylpiperidine
-
63463-36-5
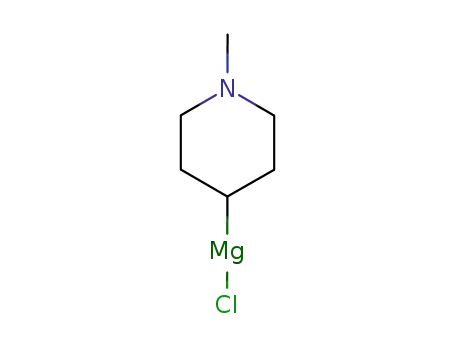
(1-methyl-4-piperidyl)magnesium chloride
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Indapamide
CAS:26807-65-8
-
Belumosudil
CAS:911417-87-3