960539-70-2
- Product Name:Daprodustat
- Molecular Formula:C19H27N3O6
- Purity:99%
- Molecular Weight:393.44
Product Details:
CasNo: 960539-70-2
Molecular Formula: C19H27N3O6
Hot Sale, Buy High Grade Daprodustat 960539-70-2 with Cheap Price
- Molecular Formula:C19H27N3O6
- Molecular Weight:393.44
- PKA:3.44±0.10(Predicted)
- PSA:124.09000
- Density:1.359±0.06 g/cm3(Predicted)
- LogP:1.52630
Daprodustat(Cas 960539-70-2) Usage
|
Description |
Daprodustat belongs to a class of drugs that regulate the body's response to low oxygen levels by stabilizing hypoxia-inducible factors (HIF). Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
| Function | Daprodustat inhibits the enzyme prolyl hydroxylase, preventing the degradation of HIF. HIF plays a crucial role in the regulation of erythropoietin (EPO), a hormone that stimulates red blood cell production. By stabilizing HIF, daprodustat increases the production of EPO, leading to enhanced erythropoiesis (red blood cell production) and increased hemoglobin levels. |
| Approval | The approval of daprodustat by regulatory agencies such as the FDA in the United States and in Japan signifies its recognition as a significant advancement in the treatment of anemia associated with CKD. Daprodustat’s cardiovascular safety has been studied extensively, showing that its risk profile is similar to that of traditional ESA therapies. This makes it a valuable option for long-term anemia management in CKD patients. |
| Uses | Daprodustat, marketed under the brand name Duvroq, is an oral medication that falls under the category of HIF-PH inhibitors. It is primarily used to treat anemia due to chronic kidney disease (CKD) in adult patients, including those undergoing dialysis. By inhibiting prolyl hydroxylase enzymes, daprodustat promotes erythropoiesis, aiding in red blood cell production in the bone marrow. Daprodustat is primarily used to treat anemia associated with CKD, a condition where the kidneys are unable to produce sufficient erythropoietin, leading to reduced red blood cell production and low hemoglobin levels. In clinical trials, daprodustat has been shown to effectively increase and maintain hemoglobin levels in CKD patients, performing comparably to traditional therapies like epoetin alfa and darbepoetin alfa. |
960539-70-2 Relevant articles
A randomized, 29-day, dose-ranging, efficacy and safety study of daprodustat, administered three times weekly in patients with anemia on hemodialysis
Christine K. Bailey, Stephen Caltabiano, Alexander R. Cobitz, Chun Huang, Kelly M. Mahar & Vickas V. Patel
, BMC Nephrology, Volume 20, article number 372, (2019)
Mean baseline hemoglobin was 10.6 g/dL for the placebo group and each daprodustat cohort. Daprodustat produced dose-dependent changes in mean hemoglobin from baseline to day 29. Using a Bayesian approach, the estimated dose conversion ratio between once-daily and TIW daprodustat was ~ 2.0 across the evaluated dose range using an Emax model. Daprodustat was generally well tolerated, with an adverse event (AE) profile consistent with the hemodialysis population.
Efficacy and Safety of Daprodustat for Treatment of Anemia of Chronic Kidney Disease in Incident Dialysis Patients
Ajay K. Singh, MBBS1,2; Borut Cizman, MD3; Kevin Carroll, PhD, CStat, CSci4
, JAMA Intern Med. 2022;182(6):592-602.
In this randomized clinical trial of 312 ID patients, daprodustat was noninferior to darbepoetin alfa in treating anemia of CKD; the difference in mean hemoglobin concentration between study arms during the evaluation period was 10.5 g/dL for patients receiving daprodustat and 10.6 g/dL for patients receiving darbepoetin alfa.
Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis
Amy M Meadowcroft, Borut Cizman, Louis Holdstock, Nandita Biswas, Brendan M Johnson, Delyth Jones, A Kaldun Nossuli, John J Lepore, Michael Aarup, Alexander R Cobitz
, Clinical Kidney Journal, Volume 12, Issue 1, February 2019, Pages 139–148
The primary outcome was characterization of the dose–response relationship between daprodustat and hemoglobin at 4 weeks; additionally, the efficacy and safety of daprodustat were assessed over 24 weeks.
960539-70-2 Upstream products
-
2387-23-7
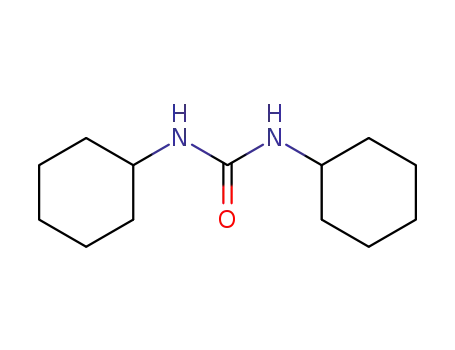
1,3-Dicyclohexylurea
-
35824-91-0
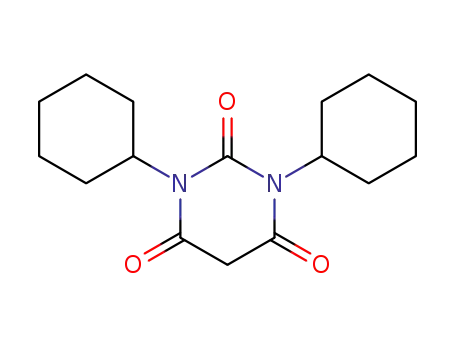
1,3-dicyclohexyl barbituric acid
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Aprocitentan
CAS:1103522-45-7
-
Encorafenib
CAS:1269440-17-6