1103522-45-7
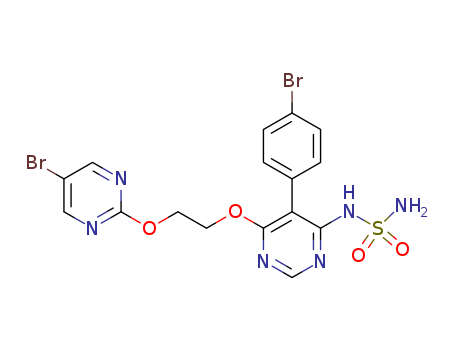
- Product Name:Aprocitentan
- Molecular Formula:C16H14Br2N6O4S
- Purity:99%
- Molecular Weight:546.199
Product Details:
CasNo: 1103522-45-7
Molecular Formula: C16H14Br2N6O4S
Hot Sale Factory Supply High Purity Aprocitentan 1103522-45-7 Lowest Price
- Molecular Formula:C16H14Br2N6O4S
- Molecular Weight:546.199
- Boiling Point:705.0±70.0 °C(Predicted)
- PKA:13.56±0.60(Predicted)
- PSA:150.59000
- Density:1.841±0.06 g/cm3(Predicted)
- LogP:4.38590
Aprocitentan(Cas 1103522-45-7) Usage
|
Description |
Aprocitentan is a sulfamide compound, with one of the amino groups substituted by a 5-(4-bromophenyl)-6-{2-[(5-bromopyrimidin-2-yl)oxy]ethoxy}pyrimidin-4-yl group. Aprocitentan (ACT-132577) is an investigational dual endothelin receptor antagonist (ERA). It acts by blocking both endothelin A (ETA) and endothelin B (ETB) receptors, preventing endothelin-1 (ET-1), a potent vasoconstrictor, from binding to these receptors. This inhibition reduces blood pressure (BP), particularly in patients with resistant hypertension, which is high blood pressure that remains uncontrolled despite the use of three or more antihypertensive medications. Aprocitentan is derived from macitentan, a drug used for pulmonary arterial hypertension. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. At present, Huarong Pharm has successfully delivered innovative R&D products and services to more than 3,000 partners across the world. |
| Uses | Aprocitentan inhibits ETA and ETB receptors, both of which mediate the vasoconstrictive and proliferative effects of ET-1. This results in vasodilation and lowered blood pressure. Aprocitentan is primarily under investigation for difficult-to-control hypertension. It has been shown to reduce blood pressure significantly in patients who do not respond well to other treatments, particularly those already on at least three antihypertensive medications, including diuretics. Clinical studies have confirmed the safety of aprocitentan, even at high doses (up to 100 mg/day), with only minor side effects observed. This supports its use for long-term management in patients needing additional BP control. |
| Resistant Hypertension Treatment | Aprocitentan represents a promising new option for patients with resistant hypertension who are not adequately controlled with standard therapies. Its ability to significantly lower BP while being well-tolerated positions it as a potential game-changer in the management of difficult-to-control hypertension. |
1103522-45-7 Relevant articles
Aprocitentan, A Dual Endothelin Receptor Antagonist Under Development for the Treatment of Resistant Hypertension
Fabio Angeli, Paolo Verdecchia & Gianpaolo Reboldi
, Cardiology and Therapy, Volume 10, pages 397–406, (2021)
Aprocitentan causes a a dose-dependent decrease in BP and has been shown to have a synergistic effect with renin-angiotensin-system (RAS) blockers. As such, it represents a potential new treatment modality when high BP cannot be adequately controlled by currently available treatments...
Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial
Prof Markus P Schlaich, MDa,b markus.schlaich@uwa.edu.au ∙ Marc Bellet, MDc ∙ Prof Michael A Weber, MDe ∙ Parisa Danaietash, PhDc ∙ Prof George L Bakris, MDf ∙ Prof John M Flack, MD
, The Lancet, 2022
The study consisted of three sequential parts: part 1 was the 4-week double-blind, randomised, and placebo-controlled part, in which patients received aprocitentan 12·5 mg, aprocitentan 25 mg, or placebo in a 1:1:1 ratio; part 2 was a 32-week single (patient)-blind part, in which all patients received aprocitentan 25 mg; and part 3 was a 12-week double-blind, randomised, and placebo-controlled withdrawal part, in which patients were re-randomised to aprocitentan 25 mg or placebo in a 1:1 ratio.
Aprocitentan, A Dual Endothelin Receptor Antagonist Under Development for the Treatment of Resistant Hypertension
Fabio Angeli, Paolo Verdecchia & Gianpaolo Reboldi
, Cardiology and Therapy, Volume 10, pages 397–406, (2021)
Significant changes in BP with aprocitentan are observed within 14 days, and its BP-lowering effects have also been documented with ambulatory BP monitoring.
1103522-45-7 Process route
-
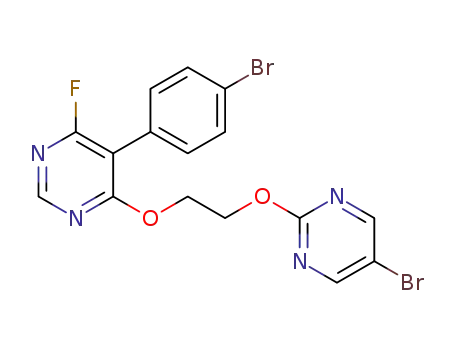
-
5-(4-bromophenyl)-4-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)-6-fluoropyrimidine

-
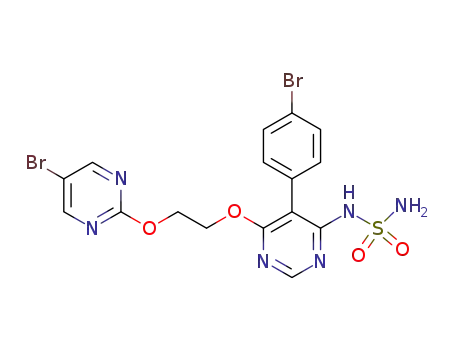
- 1103522-45-7
ACT-132577
| Conditions | Yield |
|---|---|
|
With potassium carbonate; SULFAMIDE; In dimethyl sulfoxide; at 70 ℃; for 3h; Solvent;
|
84% |
|
With potassium carbonate; SULFAMIDE; In water; dimethyl sulfoxide; at 70 - 75 ℃; for 4h; Time; Temperature;
|
77% |
|
With potassium carbonate; SULFAMIDE; In dimethyl sulfoxide; at 70 ℃; for 3h; Reagent/catalyst;
|
65% |
-
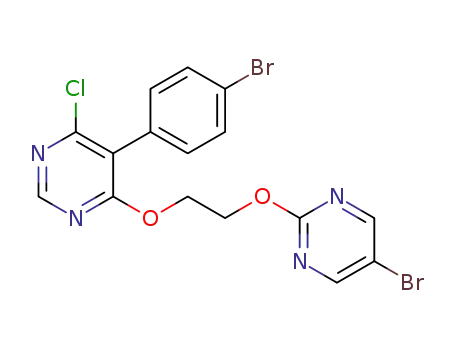
-
5-(4-bromophenyl)-4-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)-6-chloropyrimidine

-

- 1103522-45-7
ACT-132577
| Conditions | Yield |
|---|---|
|
5-(4-bromophenyl)-4-(2-((5-bromopyrimidin-2-yl)oxy)ethoxy)-6-chloropyrimidine; With cesium fluoride; In dimethyl sulfoxide; at 70 ℃; for 2.5h;
With potassium carbonate; SULFAMIDE; In dimethyl sulfoxide; for 3h; Reagent/catalyst; Heating;
|
77% |
1103522-45-7 Upstream products
-
441796-09-4
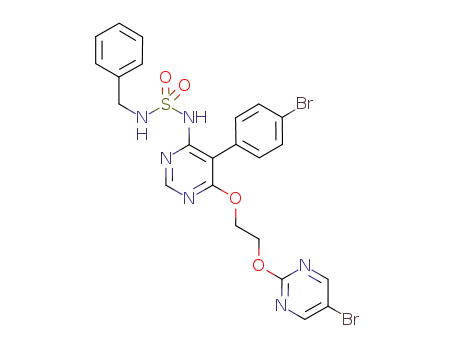
benzylsulfamic acid {5-(4-bromophenyl)-6-[2-(5-bromopyrimidin-2-yloxy)-ethoxy]-pyrimidine-4-yl}-amide
-
41841-16-1
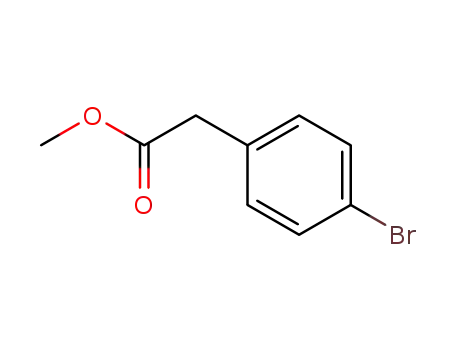
(4-bromo-phenyl)-acetic acid methyl ester
-
1878-68-8
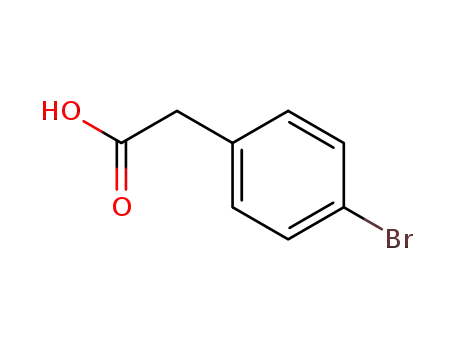
2-(4-bromophenyl)-acetic acid
-
146533-41-7
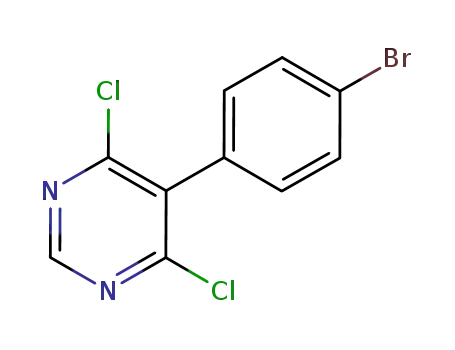
5-(4-bromophenyl)-4,6-dichloropyrimidine
Relevant Products
-
N-Bromosuccinimide
CAS:128-08-5
-
Ifosfamide
CAS:3778-73-2
-
Daprodustat
CAS:960539-70-2