3778-73-2
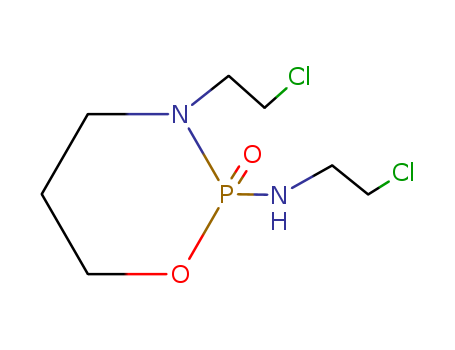
- Product Name:Ifosfamide
- Molecular Formula:C7H15Cl2N2O2P
- Purity:99%
- Molecular Weight:261.088
Product Details:
CasNo: 3778-73-2
Molecular Formula: C7H15Cl2N2O2P
Appearance: Crystalline Solid
Buy High Grade Ifosfamide,Hot Sale 3778-73-2 In Bulk Supply
- Molecular Formula:C7H15Cl2N2O2P
- Molecular Weight:261.088
- Appearance/Colour:Crystalline Solid
- Melting Point:48 °C
- Boiling Point:336.1 °C at 760 mmHg
- PKA:1.44±0.20(Predicted)
- Flash Point:157.1 °C
- PSA:51.38000
- Density:1.33 g/cm3
- LogP:2.21280
Isophosphamide(Cas 3778-73-2) Usage
|
Description |
Ifosfamide is an alkylating antitumor prodrug, similar to cyclophosphamide, and is used in the treatment of cancer. It belongs to the class of nitrogen mustard alkylating agents and is metabolized in the body to active forms that exert cytotoxic effects on cancer cells. |
|
Chemical Properties |
Crystalline Solid |
|
Originator |
Holoxan,Lucien,France,1976 |
| Uses |
Ifosfamide is indicated for the treatment of a wide range of cancers, including: Malignant lymphoma |
|
Definition |
ChEBI: The simplest member of the class of ifosfamides that is 1,3,2-oxazaphosphinan-2-amine 2-oxide substituted by 2-chloroethyl groups on both the nitrogen atoms respectively. It is a nitrogen mustard alkylating agent used in the treatment of advanced breast c ncer. |
InChI:InChI=1/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12)
3778-73-2 Relevant articles
An Overview of Cyclophosphamide and Ifosfamide Pharmacology
Ronald A. Fleming Pharm.D.
, The Journal of Human Pharmacology and Drug, Volume17, Issue5P2 September‐October 1997 Pages 146S-154S
Resistance to oxazaphosphorines is poorly understood, although increased aldehyde dehydrogenase activity may be a significant factor. Although both compounds share a common metabolic pathway, 4-hydroxylation of ifosfamide occurs at a slower rate and to a lesser extent than that of cyclophosphamide.
Phase II study of daily oral etoposide plus ifosfamide plus cisplatin for previously treated recurrent small-cell lung cancer: a Hoosier Oncology Group Trial.
EA Faylona,PJ Loehrer,R Ansari,AB Sandler,R Gonin,LH Einhorn
, Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology 1995-05-01
The study was undertaken to determine the activity and toxicity of oral etoposide (VP-16), ifosfamide, and cisplatin combination chemotherapy for previously treated, recurrent small-cell lung cancer (SCLC). In this phase II trial, 46 patients were enrolled to receive oral VP-16, 37.5 mg/m2/d for 21 days, ifosfamide 1.2 g/m2/d for 4 days, and cisplatin 20 mg/m2/d for 4 days, with courses repeated every 28 days.
No benefit of ifosfamide in Ewing's sarcoma: a nonrandomized study of the French Society of Pediatric Oncology.
O Oberlin,JL Habrand,JM Zucker,M Brunat-Mentigny,MJ Terrier-Lacombe,J Dubousset,JC Gentet,C Schmitt,D Ponvert,C Carrié
, Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology, 1992
After encouraging responses of recurrent soft tissue or bone sarcomas to ifosfamide, a second study began in 1984 using a new chemotherapy regimen in which cyclophosphamide was replaced by ifosfamide. Sixty-five patients were treated.By February 1992, the median follow-up was 5.8 years. The estimated 5-year disease-free survival was 52%.
3778-73-2 Process route
-
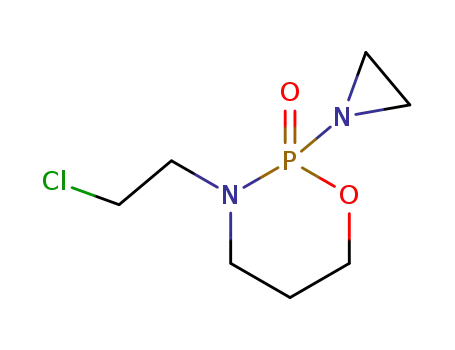
- 29102-47-4
2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide

-
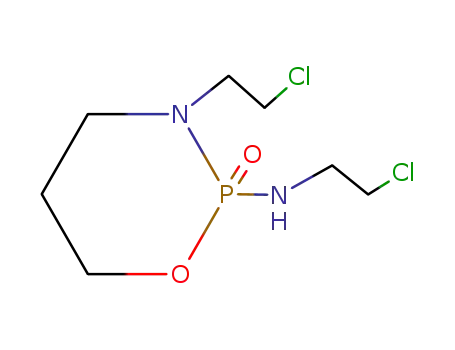
- 3778-73-2,84711-20-6
ilfosfamide
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In chloroform;
|
78% |
-
- 72578-61-1
(R)-2-aziridin-1-yl-3-((R)-1-phenyl-ethyl)-[1,3,2]oxazaphosphinane 2-oxide

-

- 3778-73-2,84711-20-6
ilfosfamide
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: aq. HCl / diethyl ether
2: H2 / Pd-C / ethanol
3: tetrahydrofuran
4: B2H6 / tetrahydrofuran
With hydrogenchloride; hydrogen; diborane; palladium on activated charcoal; In tetrahydrofuran; diethyl ether; ethanol;
|
|
|
Multi-step reaction with 4 steps
1: aq. HCl / diethyl ether
2: H2 / Pd-C / ethanol
3: tetrahydrofuran
4: B2H6 / tetrahydrofuran
With hydrogenchloride; hydrogen; diborane; palladium on activated charcoal; In tetrahydrofuran; diethyl ether; ethanol;
|
|
|
Multi-step reaction with 4 steps
1: aq. HCl / diethyl ether
2: H2 / Pd-C / ethanol
3: tetrahydrofuran
4: B2H6 / tetrahydrofuran
With hydrogenchloride; hydrogen; diborane; palladium on activated charcoal; In tetrahydrofuran; diethyl ether; ethanol;
|
|
|
Multi-step reaction with 4 steps
1: aq. HCl / diethyl ether
2: H2 / Pd-C / ethanol
3: tetrahydrofuran
4: B2H6 / tetrahydrofuran
With hydrogenchloride; hydrogen; diborane; palladium on activated charcoal; In tetrahydrofuran; diethyl ether; ethanol;
|
3778-73-2 Upstream products
-
72578-72-4
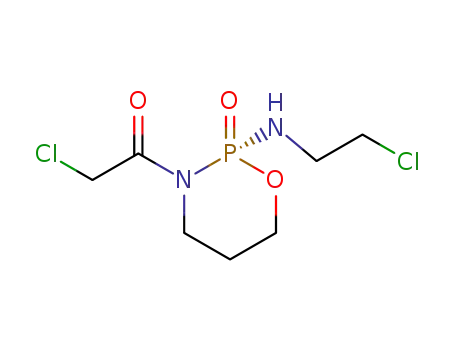
3-chloroacetyl-2-(2-chloro-ethylamino)-[1,3,2]oxazaphosphinane (S)-2-oxide
-
72578-71-3
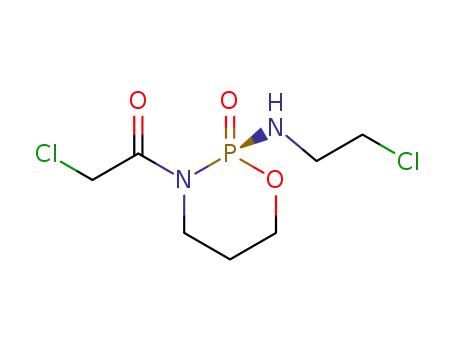
3-chloroacetyl-2-(2-chloro-ethylamino)-[1,3,2]oxazaphosphinane (R)-2-oxide
-
84681-45-8
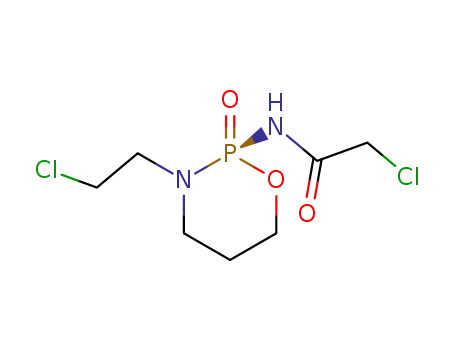
(2S)-2-<(chloroacetyl)amino>-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide
-
81485-04-3
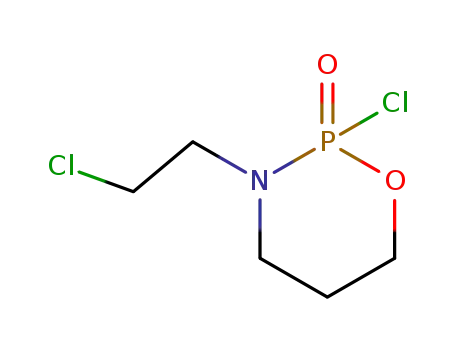
3-(chloroethyl)-2-chlorooxaazaphosphorinane 2-oxide
3778-73-2 Downstream products
-
29102-47-4

2-aziridino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin 2-oxide
-
64-17-5
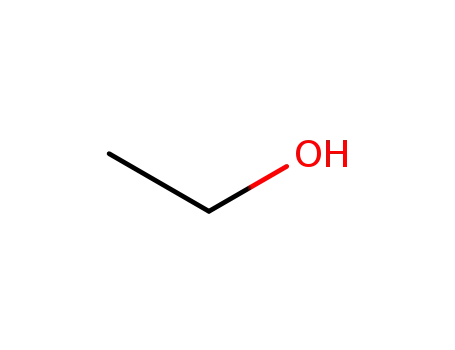
ethanol
-
689-98-5
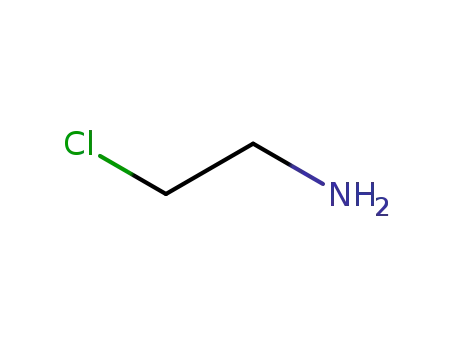
chloroethylamine
-
141056-56-6
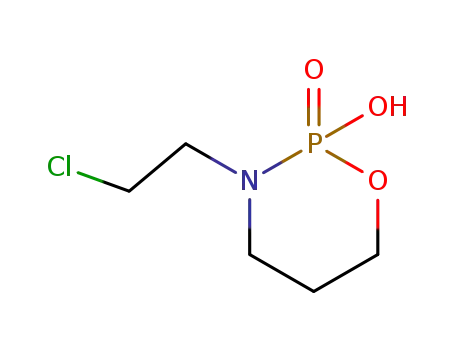
2-hydroxy-2-oxo-3-(2-chloroethyl)-1,3,2-oxazaphosphorine
Relevant Products
-
Brigatinib
CAS:1197953-54-0
-
Carfilzomib
CAS:868540-17-4
-
Aprocitentan
CAS:1103522-45-7