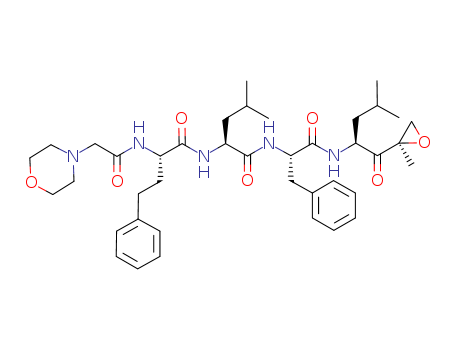
868540-17-4
- Product Name:Carfilzomib
- Molecular Formula:C40H57N5O7
- Purity:99%
- Molecular Weight:719.922
Product Details:
CasNo: 868540-17-4
Molecular Formula: C40H57N5O7
Buy Quality Carfilzomib,Export 868540-17-4 Cheap Price
- Molecular Formula:C40H57N5O7
- Molecular Weight:719.922
- Vapor Pressure:0mmHg at 25°C
- Refractive Index:1.551
- Boiling Point:975.627 °C at 760 mmHg
- PKA:13.17±0.46(Predicted)
- Flash Point:543.84 °C
- PSA:158.47000
- Density:1.162g/cm3
- LogP:4.08500
Carfilzomib(Cas 868540-17-4) Usage
| Category | Carfilzomib is classified under proteasome inhibitors, a class of drugs used primarily in cancer treatment. It specifically targets the proteasome, a protein complex responsible for degrading unwanted or damaged proteins in cells. |
| Definition | Carfilzomib is a second-generation proteasome inhibitor used to treat relapsed and refractory multiple myeloma. It selectively and irreversibly binds to the chymotrypsin-like site of the proteasome, inhibiting its activity and inducing apoptosis in cancer cells. |
|
Chemical Properties |
Solubility: Limited water solubility, used in intravenous formulations Pharmacokinetics: Rapid metabolism, primarily by peptidase cleavage and epoxide hydrolysis. Cytochrome P450 mechanisms play a minor role in its metabolism. |
|
Originator |
Proteolix Inc. (United States) |
|
Uses |
Commonly combined with dexamethasone or with lenalidomide plus dexamethasone to enhance treatment efficacy in multiple myeloma patients after 1-3 prior lines of treatment. Approved for the treatment of relapsed and refractory multiple myeloma in patients who have not responded to prior treatments. It can be used alone to treat multiple myeloma. |
|
Brand name |
Kyprolis |
| Side Effects | Common Side Effects (≥30% occurrence in trials): Fatigue, anemia, nausea, thrombocytopenia, dyspnea, diarrhea, fever. Serious Adverse Events (45% incidence): Pneumonia, acute renal failure, congestive heart failure, fever. Neuropathy: Minimal compared to other proteasome inhibitors, representing a potential advantage. |
| Clinical Studies | Carfilzomib has shown strong antitumor activity in hematologic malignancies, with a manageable safety profile, and is being studied in various other cancer types, either alone or in combination with other agents. |
InChI:InChI=1/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1
868540-17-4 Relevant articles
Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma
List of authors. A. Keith Stewart, M.B., Ch.B., S. Vincent Rajkumar, M.D., Meletios A. Dimopoulos, M.D., Tamás Masszi, M.D., Ph.D., Ivan Špička, M.D., Ph.D., Albert Oriol, M.D., Roman Hájek, M.D., Ph.D., Laura Rosiñol, M.D., Ph.D., David S. Siegel, M.D., Ph.D., Georgi G. Mihaylov, M.D., Ph.D., Vesselina Goranova-Marinova, M.D., Ph.D., Péter Rajnics, M.D., Ph.D.
N Engl J Med 2015; 372:142-152
We randomly assigned 792 patients with relapsed multiple myeloma to carfilzomib with lenalidomide and dexamethasone (carfilzomib group) or lenalidomide and dexamethasone alone (control group). The primary end point was progression-free survival.
U.S. Food and Drug Administration Approval: Carfilzomib for the Treatment of Multiple Myeloma
Thomas M. Herndon; Albert Deisseroth; Edvardas Kaminskas; Robert C. Kane; Kallappa M. Koti; Mark D. Rothmann; Bahru Habtemariam; Julie Bullock; Jeffrey D. Bray; Jessica Hawes; Todd R. Palmby; Josephine Jee; William Adams; Houda Mahayni; Janice Brown; Angelica Dorantes; Rajeshwari Sridhara; Ann T. Farrell; Richard Pazdur
Clin Cancer Res (2013) 19 (17): 4559–4563.
The safety of carfilzomib was evaluated in 526 patients with multiple myeloma treated with various dosing regimens. The ORR was 23%. The median duration of response was 7.8 months. The most common adverse reactions associated with carfilzomib infusion were fatigue, anemia, nausea, thrombocytopenia, dyspnea, diarrhea, and fever.
868540-17-4 Process route
-
![tert-butyl [(1S)-3-methyl-1-(2-methylpropyl)-2-oxobut-3-en-1-yl]carbamate](/upload/2024/1/0c20227a-8815-468f-ad28-bc4e11c8952b.png)
- 247068-81-1
tert-butyl [(1S)-3-methyl-1-(2-methylpropyl)-2-oxobut-3-en-1-yl]carbamate

-
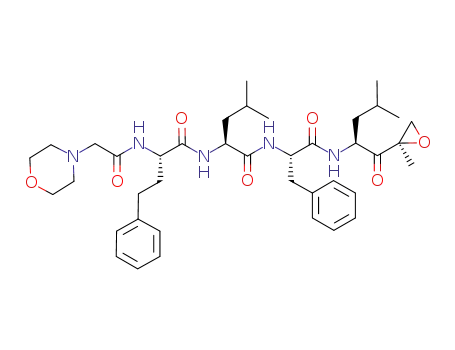
- 868540-17-4
carfilzomib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1: calcium hypochlorite / water; 1-methyl-pyrrolidin-2-one / 0.33 h / -15 - 0 °C
2: dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
3: benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1.5 h / 0 - 5 °C
With calcium hypochlorite; benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In 1-methyl-pyrrolidin-2-one; dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: sodium hypochlorite; pyridine / water / 2 h / -5 - 0 °C
2: dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere
3: benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate / N,N-dimethyl-formamide / 1.5 h / 0 - 5 °C
With pyridine; sodium hypochlorite; benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine; In dichloromethane; water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; benzonitrile; dihydrogen peroxide / methanol / 6 h / 25 - 30 °C
2: trifluoroacetic acid / dichloromethane / 3 h / 5 - 10 °C / Inert atmosphere
3: dichloromethane / 1 h
With dihydrogen peroxide; potassium carbonate; benzonitrile; trifluoroacetic acid; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; benzonitrile; dihydrogen peroxide / methanol / 6 h / 25 - 30 °C
2: trifluoroacetic acid / dichloromethane / 3 h / 5 - 10 °C / Inert atmosphere
3: dichloromethane / 3 h / 25 - 30 °C
With dihydrogen peroxide; potassium carbonate; benzonitrile; trifluoroacetic acid; In methanol; dichloromethane;
|
|
|
Multi-step reaction with 5 steps
1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4: dichloromethane / 2 h / 2 - 30 °C
5: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / 0 - 5 °C / Inert atmosphere
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3.1: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4.1: dichloromethane / 2 h / 2 - 30 °C
5.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / -10 - -5 °C
5.2: 0.5 h / 25 - 30 °C
6.1: sodium hydrogencarbonate / dichloromethane; water / 0.17 h / 2 - 6 °C
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; sodium hydrogencarbonate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; In methanol; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: cerium(III) chloride heptahydrate; sodium tetrahydroborate / methanol / 1 h / 0 - 5 °C
2.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 0 - 30 °C
3.1: Dess-Martin periodane / acetonitrile / 3 h / 0 - 30 °C
4.1: dichloromethane / 2 h / 2 - 30 °C
5.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 - 6 °C
6.1: trifluoroacetic acid / dichloromethane / 2 - 6 °C
7.1: benzotriazol-1-ol; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine / N,N-dimethyl-formamide / 2 - 6 °C
7.2: 0.5 h / 25 - 30 °C
8.1: sodium hydrogencarbonate / dichloromethane; water / 0.17 h / 2 - 6 °C
With sodium tetrahydroborate; cerium(III) chloride heptahydrate; benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; sodium hydrogencarbonate; benzotriazol-1-ol; Dess-Martin periodane; N-ethyl-N,N-diisopropylamine; 3-chloro-benzenecarboperoxoic acid; trifluoroacetic acid; In methanol; dichloromethane; water; N,N-dimethyl-formamide; acetonitrile;
|
-
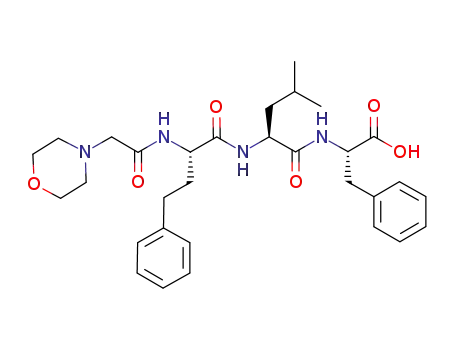
- 868540-16-3
(S)-2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenyipropanoic acid

-
-
C9H17NO2*C3HF5O2

-

- 868540-17-4
carfilzomib
| Conditions | Yield |
|---|---|
|
With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; diethylamine; In N,N-dimethyl-formamide; at -5 ℃; for 1.5h; Temperature; Reagent/catalyst;
|
86% |
868540-17-4 Upstream products
-
868540-16-3

(S)-2-((S)-4-methyl-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamido)-3-phenyipropanoic acid
-
247068-84-4
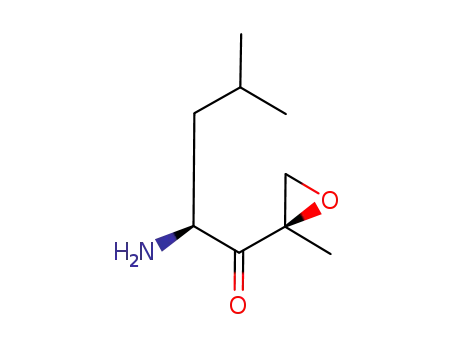
(2S)-2-amino-4-methyl-1-[(2R)-2-methyloxiran-2-yl]pentan-1-one
-
75-09-2
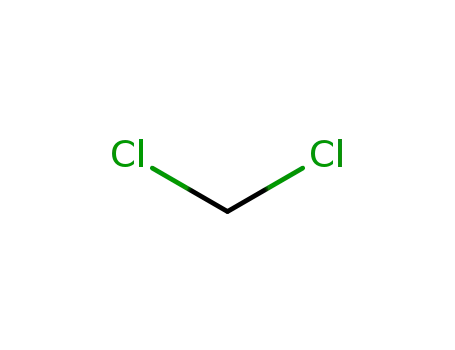
dichloromethane
-
247068-85-5
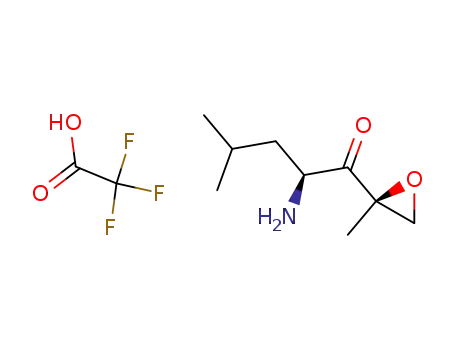
(S)-2-amino-4-methyl-1-((R)-2-methyloxiran-2-yl)pentan-1-one trifluoroacetic acid salt
Relevant Products
-
Apixaban
CAS:503612-47-3
-
(S)-(+)-3-Quinuclidinol
CAS:34583-34-1
-
Ifosfamide
CAS:3778-73-2