34583-34-1
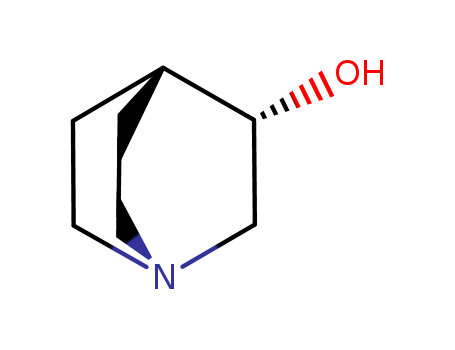
- Product Name:(S)-(+)-3-Quinuclidinol
- Molecular Formula:C7H13NO
- Purity:99%
- Molecular Weight:127.186
Product Details:
CasNo: 34583-34-1
Molecular Formula: C7H13NO
To see more products, please check our platform website--www.huarongpharm.com
Buy High Quality (S)-(+)-3-Quinuclidinol,High Purity 34583-34-1 Fast Shipping
- Molecular Formula:C7H13NO
- Molecular Weight:127.186
- Vapor Pressure:0.0545mmHg at 25°C
- Melting Point:215-217 °C
- Refractive Index:1.549
- Boiling Point:206.9 °C at 760 mmHg
- PKA:14.75±0.20(Predicted)
- Flash Point:97.7 °C
- PSA:23.47000
- Density:1.13 g/cm3
- LogP:0.01080
(S)-(+)-3-Quinuclidinol(Cas 34583-34-1) Usage
|
Description |
(S)-(+)-3-Quinuclidinol is a chiral compound with the molecular formula C7H15NO, derived from quinuclidine, a bicyclic amine. Its stereochemistry, indicated by the (S)-(+)- designation, plays a crucial role in its applications. The molecule features a quinuclidine core, comprising a five-membered ring fused to a six-membered ring. |
| Uses |
One significant application of (S)-(+)-3-Quinuclidinol is its role as an intermediate in the synthesis of Solifenacin Hydrochloride, a muscarinic M3 receptor antagonist. Solifenacin Hydrochloride is used in the treatment of overactive bladder conditions. The chiral nature of (S)-(+)-3-Quinuclidinol is particularly valuable in asymmetric synthesis, influencing the spatial arrangement of atoms in chemical reactions. Beyond its role in drug synthesis, (S)-(+)-3-Quinuclidinol has found utility in organic synthesis and medicinal chemistry. Researchers explore its derivatives for potential pharmacological properties. The compound's unique structure and stereochemistry make it versatile in various biological and chemical processes, contributing to advancements in both scientific research and industrial applications. |
InChI:InChI=1/C7H13NO/c9-7-5-8-3-1-6(7)2-4-8/h6-7,9H,1-5H2/t7-/m1/s1
34583-34-1 Relevant articles
Borneol dehydrogenase from Pseudomonas sp. TCU-HL1 possesses novel quinuclidinone reductase activities
Chen, Hao-Ping,Ho, Tsung-Jung,Hung, Chien-Chi,Khine, Aye Aye,Lu, Pei-Chieh,Simaremare, Sailent Rizki Sari,Tung, Chi-Hua,Wu, Jia-Ru,Yiin, Lin-Ming
, (2021/08/30)
Borneol dehydrogenase (BDH) catalyses th...
Preparation method of optical activity 3-quinuclidinol
-
, (2018/04/03)
The invention relates to an intermediate...
Microbial stereospecific reduction of 3-quinuclidinone with newly isolated Nocardia sp. and Rhodococcus erythropolis
Wang, Yu,Li, Jianjiong,Wu, Qiaqing,Zhu, Dunming
, p. 14 - 19 (2013/08/24)
Two bacterium strains, Nocardia sp. WY12...
A process for producing 3-quinuclidinone hydrochloride by oxidation of unwanted isomer 3-S-quinuclidinol
Chavakula, Ramadas,Rao, Mutyala Narayana,Rao, Chennupati Srinivasa
, p. 261 - 262 (2013/06/05)
An industrially efficient method was dev...
34583-34-1 Process route
-
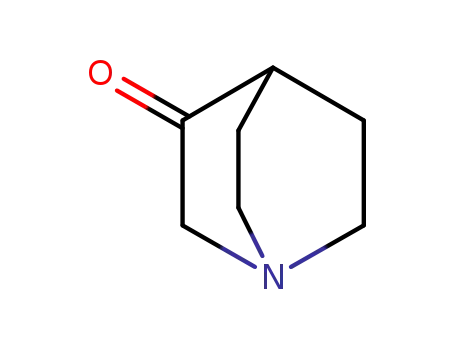
- 3731-38-2
3-Quinuclidinone

-
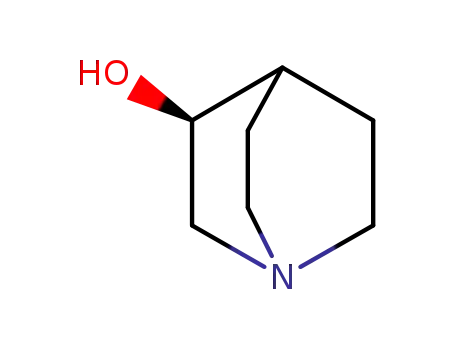
- 3684-26-2,25333-42-0,34583-34-1,1619-34-7
(S)-quinuclidin-3-ol
| Conditions | Yield |
|---|---|
|
With RuCl[(S)-daipena][(S)-3,5-xylyl-BINAP]; potassium tert-butylate; hydrogen; In isopropyl alcohol; at 10 ℃; for 6h; under 22801.5 Torr; optical yield given as %ee; enantioselective reaction;
|
97% |
|
With potassium tert-butylate; hydrogen; RuCl2[(R,R)-xylskewphos][(S)-ampy]; In ethanol; tert-butyl alcohol; at 30 ℃; for 19h; under 7600.51 Torr; Product distribution / selectivity;
|
95% |
|
With potassium tert-butylate; hydrogen; RuCl2[(R,R)-xylskewphos][(S)-ampy]; In ethanol; at 0 - 30 ℃; for 19h; under 7600.51 Torr; Product distribution / selectivity;
|
89% |
|
Multi-step reaction with 2 steps
1: lithium alanate; diethyl ether
2: (1S)-2-oxo-bornane-10-sulfonic acid
With lithium aluminium tetrahydride; diethyl ether; camphor-10-sulfonic acid;
|
|
|
With potassium tert-butylate; hydrogen; (R)-1,1'-binaphthyl-2,2'-bis(di-p-tolyl)phosphine ruthenium(II) dichloride (S,S)-1,4-diphenylbutane-1,4-diamine; In isopropyl alcohol; at 20 - 25 ℃; under 1500.15 - 15001.5 Torr; Product distribution / selectivity; Inert atmosphere; Avtoclave;
|
98 % ee |
|
With trans-RuCl2((R)-BINAP)((S)-ipban); potassium tert-butylate; hydrogen; In isopropyl alcohol; at 25 ℃; for 5h; under 15201 Torr; optical yield given as %ee; enantioselective reaction;
|
|
|
With potassium tert-butylate; hydrogen; RuCl[(S)-xylbinap][(S)-daipen]; In isopropyl alcohol; at 30 ℃; for 6h; under 22502.3 Torr; Product distribution / selectivity; Autoclave; Inert atmosphere;
|
91.2 % ee |
-
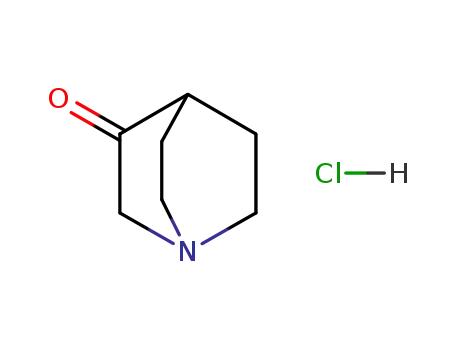
- 1193-65-3
3-quinuclidinone hydrochloride

-

- 3684-26-2,25333-42-0,34583-34-1,1619-34-7
(S)-quinuclidin-3-ol
| Conditions | Yield |
|---|---|
|
With D-glucose; In aq. phosphate buffer; at 30 ℃; for 48h; pH=8; Concentration; Temperature; pH-value; Time;
|
92% |
|
Multi-step reaction with 2 steps
1: sodium; ethanol
2: (1S)-2-oxo-bornane-10-sulfonic acid
With ethanol; camphor-10-sulfonic acid; sodium;
|
|
|
Multi-step reaction with 4 steps
1.1: sodium hydroxide; water / 0.5 h / -5 - 0 °C
1.2: 1.5 h
2.1: 2 h / 20 °C
3.1: ethanol; water / 0.33 h / 50 °C
4.1: sodium hydroxide / water / 1 h / 70 °C
With water; sodium hydroxide; In ethanol; water;
|
34583-34-1 Upstream products
-
1619-34-7
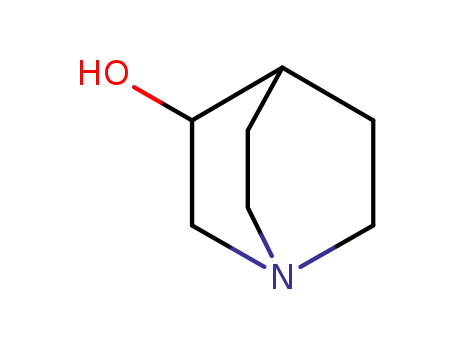
3-quinuclidinol
-
59653-42-8
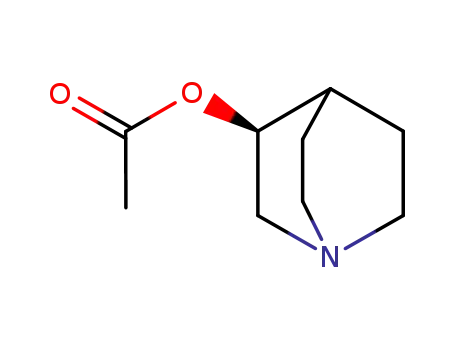
(-)-Aceclidine
-
221671-41-6
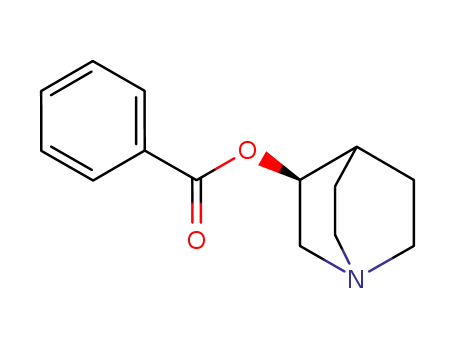
(S)-quinuclidin-3-yl benzoate
-
65732-89-0
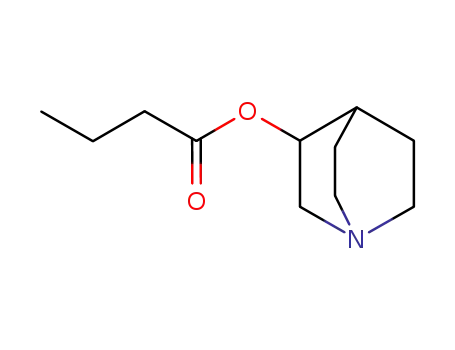
quinuclidin-3-yl butyrate b
34583-34-1 Downstream products
-
124838-74-0
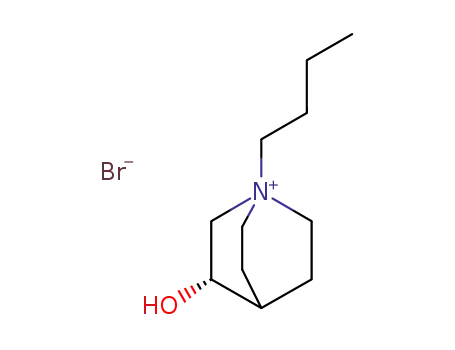
(S)-1-Butyl-3-hydroxy-1-azonia-bicyclo[2.2.2]octane; bromide
-
124838-73-9
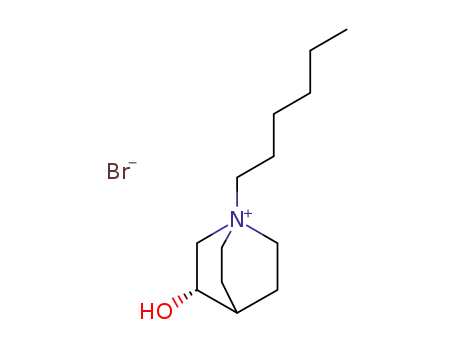
(S)-1-Hexyl-3-hydroxy-1-azonia-bicyclo[2.2.2]octane; bromide
-
124838-79-5
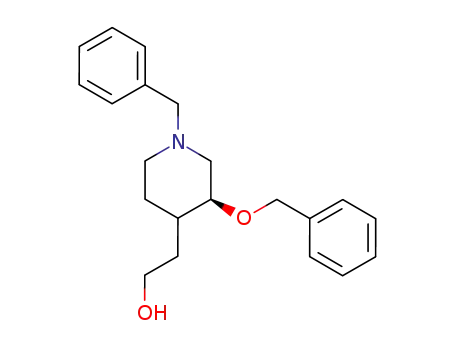
3S-benzyloxy-4RS-(2'-hydroxyethyl)-1-benzylpiperidine
-
124838-75-1
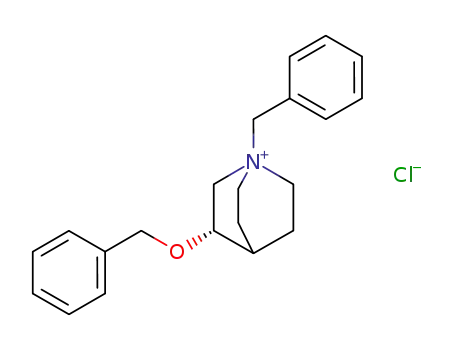
3S-benzyloxy-1-benzylquinuclidinium chloride
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Abrocitinib
CAS:1622902-68-4
-
Carfilzomib
CAS:868540-17-4