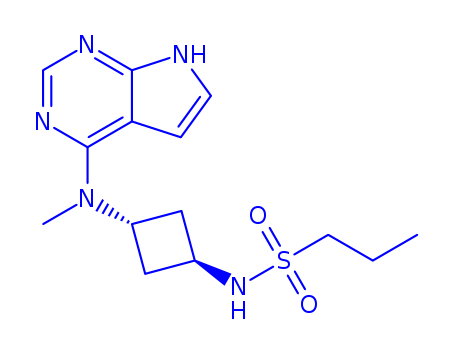
1622902-68-4
- Product Name:Abrocitinib
- Molecular Formula:C14H21N5O2S
- Purity:99%
- Molecular Weight:323.419
Product Details:
CasNo: 1622902-68-4
Molecular Formula: C14H21N5O2S
Quality Factory Supply High Purity Abrocitinib 1622902-68-4 with Safe Shipping
Molecular Formula:C14H21N5O2S
- Molecular Weight:323.419
- Density:1.36±0.1 g/cm3(Predicted)
Abrocitinib(Cas 1622902-68-4) Usage
|
Description |
Abrocitinib is an oral medication, sold under the brand name Cibinqo, used to treat moderate to severe atopic dermatitis (eczema) in adults and adolescents (12 years and older). Developed by Pfizer, Abrocitinib works by inhibiting Janus kinase 1 (JAK1), a protein involved in the immune system’s inflammatory response. This targeted inhibition helps to modulate immune signals that contribute to the pathogenesis of atopic dermatitis. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. |
| Uses | Abrocitinib is used in the treatment of moderate to severe atopic dermatitis, especially in patients who have not responded well to other treatments or cannot tolerate them. Abrocitinib is absorbed in the gastrointestinal tract and is metabolized primarily in the liver. It is taken as a once-daily oral dose. It selectively inhibits JAK1, reducing the action of inflammatory cytokines like IL-4, IL-13, IL-31, IL-22, and thymic stromal lymphopoietin. These cytokines play a key role in the inflammatory and itch response characteristic of atopic dermatitis. |
1622902-68-4 Relevant articles
Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases
Vazquez, Michael L.,Kaila, Neelu,Strohbach, Joseph W.,Trzupek, John D.,Brown, Matthew F.,Flanagan, Mark E.,Mitton-Fry, Mark J.,Johnson, Timothy A.,Tenbrink, Ruth E.,Arnold, Eric P.,Basak, Arindrajit,Heasley, Steven E.,Kwon, Soojin,Langille, Jonathan,Parikh, Mihir D.,Griffin, Sarah H.,Casavant, Jeffrey M.,Duclos, Brian A.,Fenwick, Ashley E.,Harris, Thomas M.,Han, Seungil,Caspers, Nicole,Dowty, Martin E.,Yang, Xin,Banker, Mary Ellen,Hegen, Martin,Symanowicz, Peter T.,Li, Li,Wang, Lu,Lin, Tsung H.,Jussif, Jason,Clark, James D.,Telliez, Jean-Baptiste,Robinson, Ralph P.,Unwalla, Ray
, p. 1130 - 1152 (2018)
Janus kinases (JAKs) are intracellular t...
Development of a Nitrene-Type Rearrangement for the Commercial Route of the JAK1 Inhibitor Abrocitinib
Connor, Christina G.,Deforest, Jacob C.,Dietrich, Phil,Do, Nga M.,Doyle, Kevin M.,Eisenbeis, Shane,Greenberg, Elizabeth,Griffin, Sarah H.,Jones, Brian P.,Jones, Kris N.,Karmilowicz, Michael,Kumar, Rajesh,Lewis, Chad A.,McInturff, Emma L.,McWilliams, J. Christopher,Mehta, Ruchi,Nguyen, Bao D.,Rane, Anil M.,Samas, Brian,Sitter, Barbara J.,Ward, Howard W.,Webster, Mark E.
, p. 608 - 615 (2020/10/09)
The development of a commercial route to...
Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis A Randomized Clinical Trial
Jonathan I. Silverberg, MD, PhD, MPH1; Eric L. Simpson, MD2; Jacob P. Thyssen, MD3
, Original Investigation June 3, 2020
A total of 391 patients (229 male [58.6%]; mean [SD] age, 35.1 [15.1] years) were included in the analysis; of these, 155 received abrocitinib, 200 mg/d; 158, abrocitinib, 100 mg/d; and 78, placebo. Among patients with available data at week 12, greater proportions of patients in the 200- and 100-mg abrocitinib groups vs the placebo group achieved IGA (59 of 155 [38.1%] and 44 of 155 [28.4%] vs 7 of 77 [9.1%]; P < .001) and EASI-75 (94 of 154 [61.0%] and 69 of 155 [44.5%] vs 8 of 77 [10.4%]; P < .001), greater estimated proportions achieved PP-NRS (55.3% [95% CI, 47.2%-63.5%] and 45.2% [95% CI, 37.1%-53.3%] vs 11.5% [95% CI, 4.1%-19.0%]; P < .001), and/or greater proportions achieved EASI-90 (58 of 154 [37.7%] and 37 of 155 [23.9%] vs 3 of 77 [3.9%]) responses.
Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib
Novin Nezamololama, BSc, MSc,1 Keira Fieldhouse, BSc,1,2 Kristy Metzger, BSc,1,2 and Melinda Gooderham, MD, MSc, FRCPCcorresponding author1,3,4
Drugs Context. 2020; 9: 2020-8-5.
This review summarizes the clinical data available from various trials and reports on the safety and efficacy of abrocitinib, baricitinib, and upadacitinib, the three oral systemic JAK inhibitors used in the treatment of AD. The safety and efficacy of JAK inhibitors for the treatment of AD are emerging in the literature.
1622902-68-4 Process route
-
![N-(cis-3-(methyl(7-tosyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide](/upload/2024/1/cf85ebf2-c453-4a4a-87b4-89ef5143aee7.png)
-
N-(cis-3-(methyl(7-tosyl-7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)cyclobutyl)propane-1-sulfonamide

-
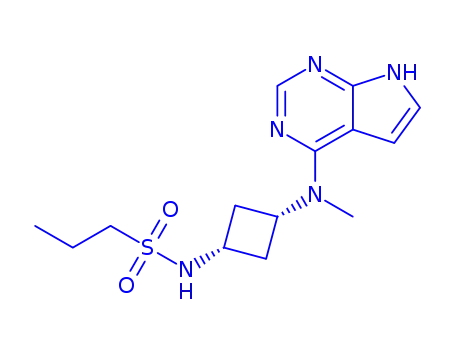
- 1622902-68-4
PF-04965842
| Conditions | Yield |
|---|---|
|
With lithium hydroxide monohydrate; water; In isopropyl alcohol; at 60 ℃; for 13h;
|
35% |
|
With sodium hydroxide; In 2-methyltetrahydrofuran; water; for 1h; Inert atmosphere; Reflux;
|
56.2 g |
|
With sodium hydroxide; In water; at 70 ℃; for 4h;
|
5.39 g |
-
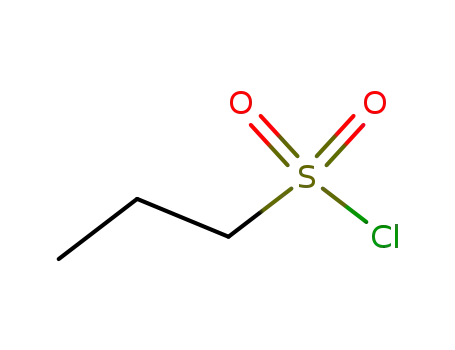
- 10147-36-1
n-propanesulfonyl chloride

-
-
(1s,3s)-N1-methyl-N1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)cyclobutane-1,3-diamine phosphoric acid

-

- 1622902-68-4
PF-04965842
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In 2-methyltetrahydrofuran; water; at 10 - 20 ℃; pH=2;
|
96% |
1622902-68-4 Upstream products
-
90213-66-4
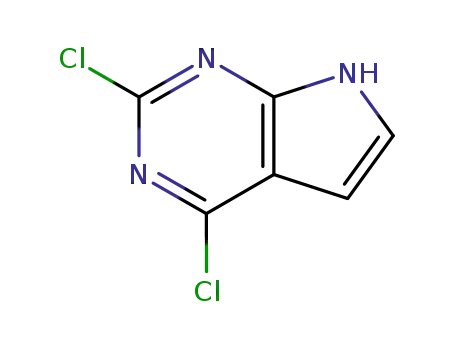
2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine
-
479633-63-1
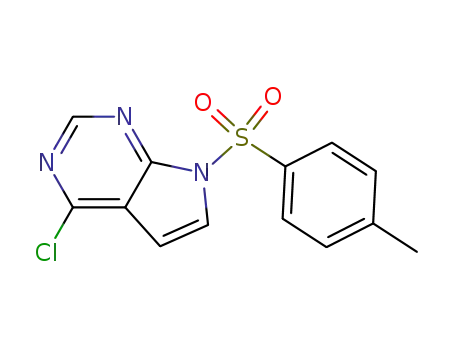
4-chloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine
-
10147-36-1

n-propanesulfonyl chloride
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
LCZ696
CAS:936623-90-4
-
(S)-(+)-3-Quinuclidinol
CAS:34583-34-1