936623-90-4
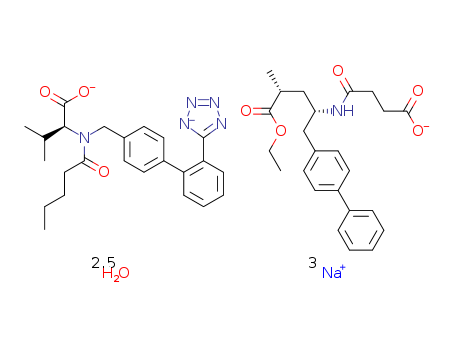
- Product Name:LCZ696
- Molecular Formula:C49H59N6Na3O8
- Purity:99%
- Molecular Weight:958.008
Product Details:
CasNo: 936623-90-4
Molecular Formula: C49H59N6Na3O8
Buy High Grade LCZ696,Offer 936623-90-4 Low Price
- Molecular Formula:2(C24H29N5O3).2(C24H29NO5).5(H2O).6Na
- Molecular Weight:958.008
- PSA:207.53000
- LogP:5.98340
LCZ696 (Cas 936623-90-4) Usage
|
Description |
LCZ696, marketed under the brand name Entresto™ by Novartis, is a first-in-class angiotensin II receptor-neprilysin inhibitor (ARNI). It is used in the treatment of heart failure (HF) and arterial hypertension due to its unique mode of action that targets both the renin-angiotensin-aldosterone system (RAAS) and the natriuretic peptide system. LCZ696, also known by the brand name Entresto, is a combination drug that is synthesized using a variety of methods, including:Convergent synthesis, Flow reactor and Chiral induction reagent. |
| Uses |
LCZ696, a combination of sacubitril and valsartan, works by inhibiting neprilysin and the angiotensin type 1 (AT1) receptor. Heart Failure: LCZ696 is indicated for the treatment of heart failure with reduced ejection fraction (HFrEF). It has been shown to be superior to enalapril, an angiotensin-converting enzyme (ACE) inhibitor, in reducing the risk of cardiovascular death and heart failure hospitalization. The most common side effects of LCZ696, also known as Entresto, include: low blood pressure, high potassium levels, cough, dizziness, and kidney problems. |
| Pharmacokinetics | Absorption: LCZ696 is rapidly absorbed after oral administration, with peak plasma concentrations reached within hours for both valsartan and sacubitril. Metabolism: Sacubitril is metabolized into its active moiety, LBQ657, by esterases. Excretion: LCZ696 and its metabolites are excreted primarily through renal and biliary routes. |
936623-90-4 Relevant articles
The Antihypertensive Effects and Safety of LCZ696 in Patients with Hypertension: A Systemic Review and Meta-Analysis of Randomized Controlled Trials
Su-Kiat Chua 1,2,3,†ORCID,Wei-Ting Lai 2,3,†,Lung-Ching Chen 2,3 andHuei-Fong Hung 2,3,*
, J. Clin. Med. 2021, 10(13), 2824;
The inclusion criteria for this network meta-analysis were as follows: (1) all relevant Phase 3 RCTs comparing LCZ696 and placebo in patients with hypertension; (2) reported mean systolic blood pressure (msSBP) and mean diastolic blood pressure (msDBP) in the sitting position; (3) reported mean ambulatory systolic blood pressure (maSBP) and mean ambulatory diastolic blood pressure (maDBP); (4) reported trial-defined adverse events...
Pharmacokinetics and Pharmacodynamics of LCZ696, a Novel Dual-Acting Angiotensin Receptor—Neprilysin Inhibitor (ARNi)
Dr Jessie Gu PhD, Dr Adele Noe PhD, Dr Priya Chandra PhD, Dr Suliman Al-Fayoumi PhD, Dr Monica Ligueros-Saylan MD, Dr Ramesh Sarangapani PhD, MBA, Dr Suzanne Maahs PharmD, Dr Gary Ksander PhD, Dr Dean F. Rigel PhD, Dr Arco Y. Jeng PhD, Dr Tsu-Han Lin PhD, Dr Weiyi Zheng PhD, Dr William P. Dole MD
The Journal of Clinical Pharmacology, Volume50, Issue4 April 2010 Pages 401-414
LCZ696 treatment was associated with increases in plasma cGMP, renin concentration and activity, and angiotensin II, providing evidence for NEP inhibition and angiotensin receptor blockade. In a randomized, open-label crossover study in healthy participants (n = 56), oral LCZ696 400 mg and valsartan 320 mg were shown to provide similar exposure to valsartan (geometric mean ratio [90% confidence interval]: AUC0-∞ 0.90 [0.82–0.99]).
Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study
Prof Luis Miguel Ruilope, MD Prof Andrej Dukat, MD Prof Michael Böhm, MD Yves Lacourcière, MD Jianjian Gong, PhD Martin P Lefkowitz, MD
The Lancet, VOLUME 375, ISSUE 9722, P1255-1266, APRIL 10, 2010
The primary endpoint was the mean difference across the three single-dose pairwise comparisons of LCZ696 versus valsartan (100 mg vs 80 mg, 200 mg vs 160 mg, and 400 mg vs 320 mg) in mean sitting diastolic blood pressure during the 8-week treatment period. Analysis was by intention to treat.
936623-90-4 Process route
-
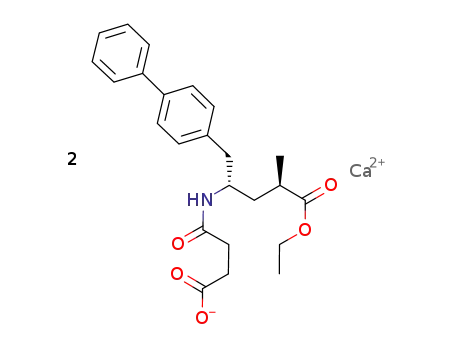
-
N-(3-carboxyl-1-oxopropyl)-(4S)-(p-phenylphenyl-methyl)-4-amino-(2R)-methylbutanoic acid ethyl ester calcium salt

-
![N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine](/upload/2024/1/01404fed-c3dc-4196-9731-3f73e738e728.png)
- 137862-53-4,137862-87-4
N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine

-
![trisodium [3-((1S,3R)-1-biphenyl-4-yl-methyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3’-methyl-2’-(pentanoyl{2”-(tetrazol-5-ylate)biphenyl-4’-yl-methyl}amino)butyrate] hemipentahydrate](/upload/2024/1/a7dff434-7798-42fd-a9f3-9ff97d7bb310.png)
- 936623-90-4
trisodium [3-((1S,3R)-1-biphenyl-4-yl-methyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3’-methyl-2’-(pentanoyl{2”-(tetrazol-5-ylate)biphenyl-4’-yl-methyl}amino)butyrate] hemipentahydrate
| Conditions | Yield |
|---|---|
|
N-(3-carboxyl-1-oxopropyl)-(4S)-(p-phenylphenyl-methyl)-4-amino-(2R)-methylbutanoic acid ethyl ester calcium salt; With hydrogenchloride; water; In Isopropyl acetate;
N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine; With sodium hydroxide; In n-heptane; water; acetone; at 45 ℃; Temperature;
|
91.9% |
-
![4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoic acid](/upload/2024/1/a18e0034-c987-4c3f-a6d8-fdf455bd5edd.png)
- 149709-63-7,766480-48-2
4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoic acid

-
![N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine](/upload/2024/1/01404fed-c3dc-4196-9731-3f73e738e728.png)
- 137862-53-4,137862-87-4
N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine

-
![trisodium [3-((1S,3R)-1-biphenyl-4-yl-methyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3’-methyl-2’-(pentanoyl{2”-(tetrazol-5-ylate)biphenyl-4’-yl-methyl}amino)butyrate] hemipentahydrate](/upload/2024/1/a7dff434-7798-42fd-a9f3-9ff97d7bb310.png)
- 936623-90-4
trisodium [3-((1S,3R)-1-biphenyl-4-yl-methyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-(S)-3’-methyl-2’-(pentanoyl{2”-(tetrazol-5-ylate)biphenyl-4’-yl-methyl}amino)butyrate] hemipentahydrate
| Conditions | Yield |
|---|---|
|
With sodium hydroxide; In water; acetone; at 25 - 35 ℃; for 1h;
|
90.83% |
936623-90-4 Upstream products
-
137862-53-4
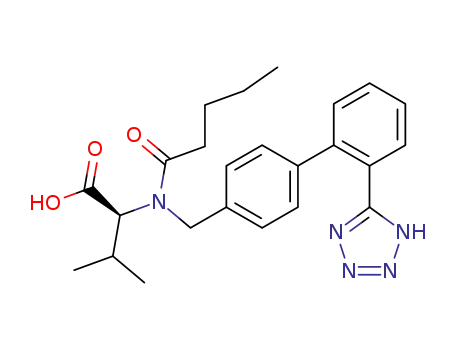
N-pentanoyl-N-[[2'-(1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]-methyl]-(L)-valine
-
149709-63-7
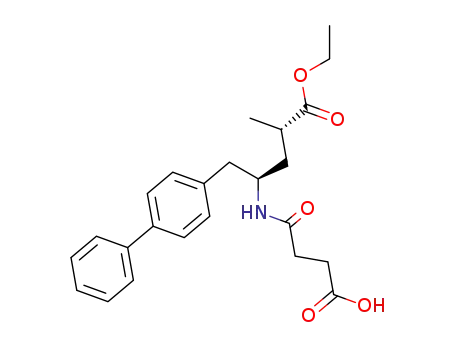
4-(((2S,4R)-1-([1,1'-biphenyl]-4-yl)-5-ethoxy-4-methyl-5-oxopentan-2-yl)amino)-4-oxobutanoic acid
-
149709-62-6
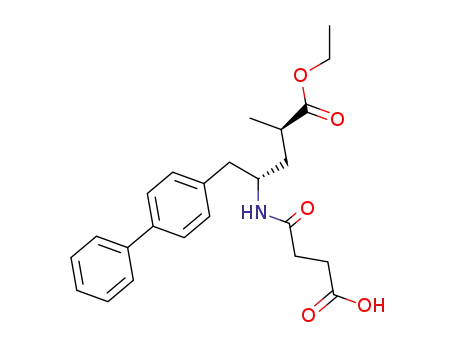
α-ethyl (αR,γS)-γ-<(3-carboxy-1-oxopropyl)amino>-α-methyl<1,1'-biphenyl>-4-pentanoate
Relevant Products
-
Nemorexant
CAS:1505484-82-1
-
Denosumab
CAS:615258-40-7
-
Abrocitinib
CAS:1622902-68-4