1505484-82-1
- Product Name:Nemorexant
- Molecular Formula:C23H23ClN6O2
- Purity:99%
- Molecular Weight:450.928
Product Details:
CasNo: 1505484-82-1
Molecular Formula: C23H23ClN6O2
Factory Supply High Purity Nemorexant 1505484-82-1,Factory Sells 1505484-82-1 Best Price
- Molecular Formula:C23H23ClN6O2
- Molecular Weight:450.928
- Boiling Point:747.6±70.0 °C(Predicted)
- Density:1.42±0.1 g/cm3(Predicted)
Nemorexant(Cas 1505484-82-1) Usage
|
Description |
Nemeroxant is a selective dual orexin receptor antagonist (DORA) that is used to treat insomnia. It is also known by the name daridorexant. |
| Uses |
Daridorexant, formerly known as nemorexant, is a selective dual orexin receptor antagonist used to treat insomnia. Insomnia is characterized by difficulties with sleep onset and/or sleep maintenance and impairment of daytime functioning. |
1505484-82-1 Relevant articles
CYP3A4 Catalyzes the Rearrangement of the Dual Orexin Receptor Antagonist Daridorexant to 4‐Hydroxypiperidinol Metabolites
A Treiber,H Aissaoui,S Delahaye,S Glutz,J Grimont,C Müller,S Seeland,V Siefken,C Boss
, ChemMedChem, 2023
With human liver microsomes, daridorexant underwent hydroxylation at the methyl group of the benzimidazole moiety, oxidative O‐demethylation of the anisole to the corresponding phenol, and hydroxylation to a 4‐hydroxy piperidinol derivative.
The Quest for the Best Dual Orexin Receptor Antagonist (Daridorexant) for the Treatment of Insomnia Disorders
Boss, Christoph,Gatfield, John,Brotschi, Christine,Heidmann, Bibia,Sifferlen, Thierry,von Raumer, Markus,Schmidt, Gunther,Williams, Jodi T.,Treiber, Alexander,Roch, Catherine
, p. 2286 - 2305 (2020)
Since its discovery in 1998, the orexin ...
1505484-82-1 Process route
-
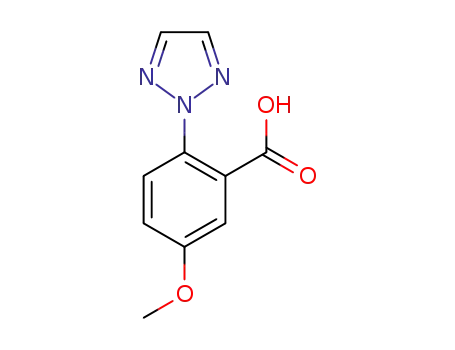
- 1293284-55-5
5-methoxy-2-(2H-1,2,3-triazol-2-yl)benzoic acid

-
-
C13H16ClN3*ClH

-
![(S)-(2-(6-chloro-7-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone](/upload/2024/1/641786a3-5255-4525-a5d1-cd6c7d49bf91.png)
- 1505484-82-1
(S)-(2-(6-chloro-7-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone
| Conditions | Yield |
|---|---|
|
With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In dichloromethane; at 20 ℃; for 16h;
|
63% |
-
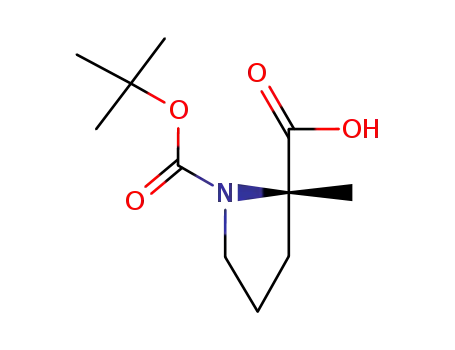
- 103336-06-7
N-(tert-butoxycarbonyl)-2-methyl-L-proline

-
![(S)-(2-(6-chloro-7-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone](/upload/2024/1/641786a3-5255-4525-a5d1-cd6c7d49bf91.png)
- 1505484-82-1
(S)-(2-(6-chloro-7-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5-methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / dichloromethane / 16 h / 20 °C
1.2: 1 h / 100 °C
2.1: hydrogenchloride / 1,4-dioxane / 1.5 h / 20 °C
3.1: N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate / dichloromethane / 16 h / 20 °C
With hydrogenchloride; N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In 1,4-dioxane; dichloromethane;
|
1505484-82-1 Upstream products
-
103336-06-7

N-(tert-butoxycarbonyl)-2-methyl-L-proline
-
673487-36-0
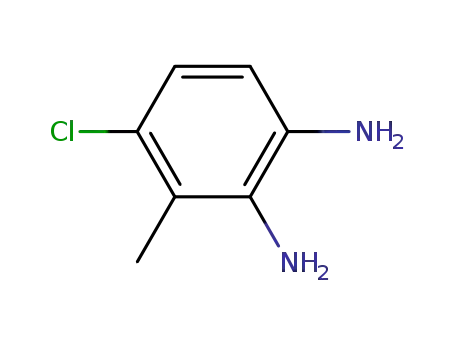
4-chloro-3-methylbenzene-1,2-diamine
-
1293284-55-5

5-methoxy-2-(2H-1,2,3-triazol-2-yl)benzoic acid
-
54413-93-3
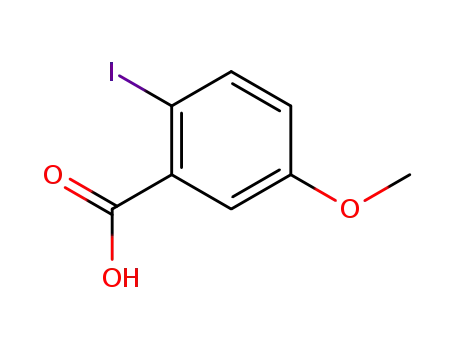
2-iodo-5-methoxybenzoic acid
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Moxifloxacine Hcl
CAS:186826-86-8
-
Lacosamide
CAS:175481-36-4