186826-86-8
- Product Name:Moxifloxacine Hcl
- Molecular Formula:C21H24FN3O4.ClH
- Purity:99%
- Molecular Weight:437.899
Product Details:
CasNo: 186826-86-8
Molecular Formula: C21H24FN3O4.ClH
Appearance: 99%
Quality Factory Supply 186826-86-8 Reasonable Price, Sale Moxifloxacine Hcl
- Molecular Formula:C21H24FN3O4.ClH
- Molecular Weight:437.899
- Appearance/Colour:99%
- Vapor Pressure:4.56E-17mmHg at 25°C
- Melting Point:Slightly yellow to yellow crystalline powder, mp 324-325°
- Boiling Point:636.4 °C at 760 mmHg
- Flash Point:338.7 °C
- PSA:83.80000
- LogP:3.56630
Moxifloxacin hydrochloride(Cas 186826-86-8) Usage
|
Description |
Moxifloxacin hydrochloride is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of certain bacterial infections, such as community-acquired pneumonia, acute worsening of chronic bronchitis, acute sinus infections, plague, and skin and abdominal infections. |
| Appearance | Slightly yellow to yellow crystalline substance |
| Synthesis | Racemic 2,8-diazabicyclo[4.3.0]nonane is prepared, then resolved with tartaric acid. A quinolinecarboxylic acid is then introduced, and acidification forms moxifloxacin hydrochloride |
|
Uses |
Moxifloxacin HCl is a broad-spectrum fluoroquinolone antibiotic used to treat various bacterial infections. It is particularly effective against respiratory tract infections, including pneumonia, bronchitis, and sinusitis, as well as skin and soft tissue infections. Moxifloxacin HCl works by inhibiting bacterial DNA gyrase and topoisomerase IV, enzymes crucial for DNA replication and repair, leading to the death of the bacteria. It is also used in the treatment of certain eye infections, such as bacterial conjunctivitis, in the form of eye drops. Due to its broad-spectrum activity, it is often reserved for use when other antibiotics are ineffective or contraindicated. |
| Quality Factory | Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
InChI:InChI=1/C21H24FN3O4.ClH/c1-29-20-17-13(19(26)14(21(27)28)9-25(17)12-4-5-12)7-15(22)18(20)24-8-11-3-2-6-23-16(11)10-24;/h7,9,11-12,16,23H,2-6,8,10H2,1H3,(H,27,28);1H
186826-86-8 Relevant articles
Review of moxifloxacin hydrochloride ophthalmic solution in the treatment of bacterial eye infections
Darlene Miller
, Clinical Ophthalmology Volume 2, 2008 - Issue 1
Moxifloxacin hydrochloride ophthalmic solution 0.5%, under the trade name Vegamox®, was introduced into Japan in 2006, with approval for the treatment of bacterial conjunctivitis, keratitis and surgical prophylaxis.
Sensitive Determination of Moxifloxacin HCl in Pharmaceuticals or Human Plasma Using Luminescence or Eye Vision
Gasser M. Khairy 1,*ORCID,Zaitona A. Abd El-Naby 2,Alaa M. A. Elgindy 2,Axel Duerkop 3,*ORCID andEman A. Abdel Hameed 4
, Chemosensors 2022, 10(10), 378;
A new probe based on the complex of 1,2 dihydro-2-oxoquinoloine-4-carboxylic acid (DOCA) as a ligand with Europium (III) ion was developed for the quantitation of Moxifloxacin HCl (Moxi.HCl) in pharmaceuticals and human plasma using a luminescence method. The metal to ligand ratio of the complex is 1:2 as determined by a Job plot. The determination of Moxi.HCl is based on static quenching of the luminescence of the probe upon coordination of Moxi.HCl.
Validated spectrophotometric methods for the estimation of moxifloxacin in bulk and pharmaceutical formulations
Sanjay K. Motwani, Shruti Chopra 1, Farhan J. Ahmad 1, Roop K. Khar 1
, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Volume 68, Issue 2, October 2007, Pages 250-256
Moxifloxacin is a fourth generation 8-methoxy fluoroquinolone derivative [1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-{(4aS,7aS–octa-hydro-6H-pyrrolol (3,4b) pyridin-6-yl)}-4-oxo-3-quinoline carboxylic acid, monohydrochloride] with extended-spectrum and improved activity against Gram-positive bacteria (including staphylococci, streptococci, enterococci), anaerobes and atypical bacteria
186826-86-8 Process route
-
-
(4aS-cis)-1-cyclopropyl-7-(2,8-diazabicyclo-[4.3.0]non-8-yl)-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinoline carboxylic acid-O3,O4(bis(acyloxy-O)) borate

-
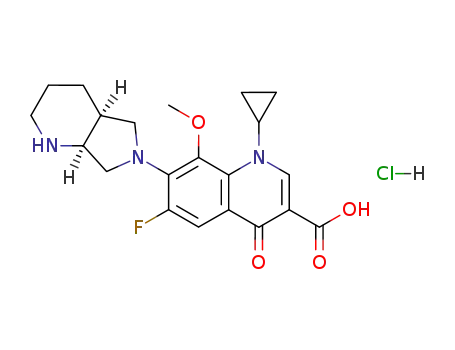
- 186826-86-8,1246654-75-0,1346603-25-5
moxifloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; water; at 0 - 8 ℃; for 0.333333h; Temperature;
|
96.2% |
|
With hydrogenchloride; In water; at 0 ℃; for 4h; pH=< 1;
|
96.5% |
|
With hydrogenchloride; In methanol; at -5 - 20 ℃; for 2h; pH=1; Temperature;
|
93.1% |
|
With sodium hydroxide; In acetone; for 1h; Large scale;
|
89.8% |
|
With sodium hydroxide; at 80 ℃; for 2.5h;
|
84.75% |
|
With hydrogenchloride; In water; at 10 - 15 ℃; for 6h; pH=1.0 - 2.0;
|
118 g |
|
With hydrogenchloride; In ethanol; water; at 25 ℃; for 1h; pH=1; Temperature;
|
48 g |
-
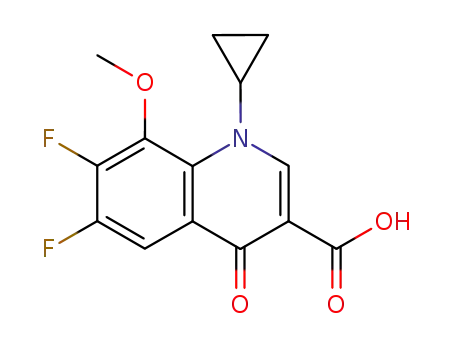
- 112811-72-0
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid

-
![(1S,6S)-2,8-diazabicyclo[4.3.0]nonane](/upload/2024/1/247cf9a1-264f-4309-b591-2575cd71a4f8.png)
- 151213-40-0,158060-81-2,169533-56-6
(1S,6S)-2,8-diazabicyclo[4.3.0]nonane

-

- 186826-86-8,1246654-75-0,1346603-25-5
moxifloxacin hydrochloride
| Conditions | Yield |
|---|---|
|
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid; (1S,6S)-2,8-diazabicyclo[4.3.0]nonane; With titanium(IV) isopropylate; triethylamine; In isopropyl alcohol; at 100 ℃; Inert atmosphere;
With hydrogenchloride; In methanol; at 20 ℃; for 1h; pH=1; Solvent; Reagent/catalyst;
|
90% |
|
Multi-step reaction with 2 steps
1: Alkaline conditions
2: hydrogenchloride
With hydrogenchloride;
|
|
|
1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid; (1S,6S)-2,8-diazabicyclo[4.3.0]nonane; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In acetonitrile; at 20 - 85 ℃; for 36h; Inert atmosphere;
With hydrogenchloride; In water; at 15 ℃; for 1h; pH=1.4 - 1.8; Inert atmosphere;
|
3.3 g |
186826-86-8 Upstream products
-
151213-40-0
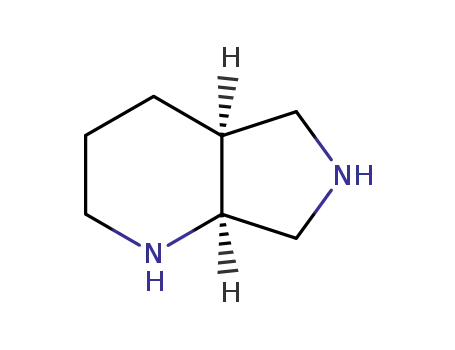
(1S,6S)-2,8-diazabicyclo[4.3.0]nonane
-
151096-09-2
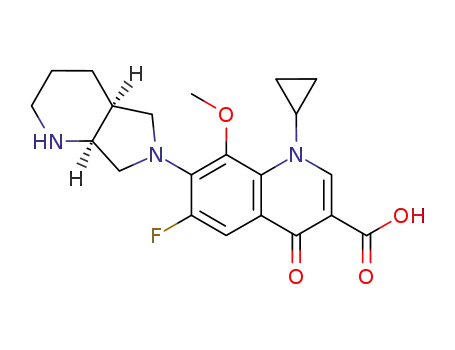
moxifloxacin
-
192927-63-2
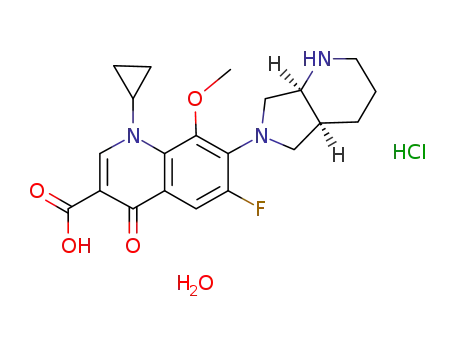
1-cyclopropyl-7-([S,S]-2,8-diazabicyclo[4.3.0]non-8-yl)-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acid monohydrochloride monohydrate
-
496919-99-4
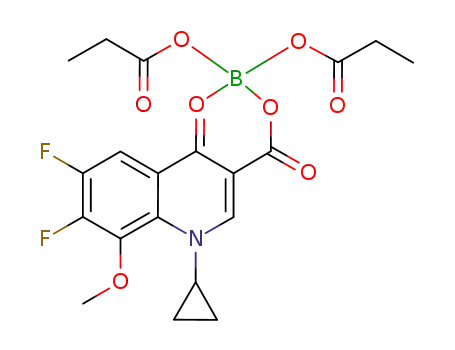
1-cyclopropyl-6,7-difluoro-8-methoxy-4-oxo-1,4-dihydro-3-quinoline-carboxylic acid O3,O4-bis(propyloxy-O)borate
186826-86-8 Downstream products
-
151096-09-2

moxifloxacin
Relevant Products
-
Nebivolol hcl
CAS:152520-56-4
-
Tirofiban Hcl
CAS:142373-60-2
-
Nemorexant
CAS:1505484-82-1