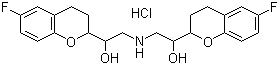
152520-56-4
- Product Name:Nebivolol hcl
- Molecular Formula:C22H25F2NO4.HCl
- Purity:99%
- Molecular Weight:441.90
Product Details:
CasNo: 152520-56-4
Molecular Formula: C22H25F2NO4.HCl
Appearance: White to off-white powder
Buy Quality Nebivolol hcl,Wholesale 152520-56-4 In Bulk Supply
- Molecular Formula:C22H25F2NO4.HCl
- Molecular Weight:441.90
- Appearance/Colour:White to off-white powder
- Vapor Pressure:2.88E-15mmHg at 25°C
- Melting Point:220-222 °C
- Boiling Point:600.5 °C at 760 mmHg
- Flash Point:316.9 °C
- PSA:70.95000
- LogP:3.55650
Nebivolol hydrochloride(Cas 152520-56-4) Usage
|
Chemical Properties |
White to Off-White Powder |
|
Description |
Nebivolol hydrochloride is a BCS Class II drug (low solubility, high permeability). It has a crystalline structure that enables the formation of cocrystals with other compounds like 4-hydroxybenzoic acid and nicotinamide, improving its solubility and dissolution rate. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
| Uses |
Nebivolol hydrochloride is categorized as a beta-1 adrenergic receptor blocker (β1-blocker), primarily used as an antihypertensive agent. Nebivolol hydrochloride is a highly selective beta-1 adrenergic receptor antagonist, used for the management of hypertension. It works by blocking the effects of epinephrine on the heart, leading to a slower heart rate and the relaxation of blood vessels, thus reducing blood pressure and improving blood flow. Pharmacokinetics: |
InChI:InChI=1/C22H25F2NO4.ClH/c23-15-3-7-19-13(9-15)1-5-21(28-19)17(26)11-25-12-18(27)22-6-2-14-10-16(24)4-8-20(14)29-22;/h3-4,7-10,17-18,21-22,25-27H,1-2,5-6,11-12H2;1H
152520-56-4 Relevant articles
Spectrophotometric Method for the Determination of Nebivolol Hydrochloride in Bulk and Pharmaceutical Formulations
A. LAKSHMANA RAO* , K.R. RAJESWARI and G.G. SANKAR
E-Journal of Chemistry, 2010
A simple, sensitive, highly accurate spectrophotometric method in UV region has been developed for the determination of nebivolol hydrochloride in bulk and pharmaceutical formulations. Nebivolol hydrochloride is an antihypertensive drug, which shows maximum absorbance at 281 nm with apparent molar absorptivity of 5.37208 x 103 mol-1 cm-1. Beer’s law was obeyed in the concentration range of 4-60 µg/mL. The slope, intercept and correlation coefficient were also calculated.
Development and Validation for the Simultaneous Quantification of Nebivolol Hydrochloride and Hydrochlorothiazide by UV Spectroscopy, RP-HPLC and HPTLC in Tablets
B. Dhandapani ,1N. Thirumoorthy,2and D. Jose Prakash3
Journal of Chemistry, 2010, Volume 7
Simultaneous quantification of nebivolol hydrochloride (NEB-H) and hydrochlorothiazide (HCT) in tablets by UV spectroscopy, RP-HPLC and HPTLC methods were developed. In UV spectrophotometric determination NEB-H and HCT was quantified by simultaneous equation method and absorbance ratio method. In simultaneous equation method absorbance measurements at 282.5 nm (λmax NEB-H) and 271.5 nm (λmax HCT),
152520-56-4 Upstream products
-
212909-96-1
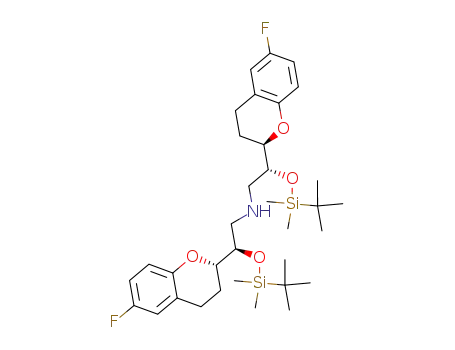
[(R)-2-(tert-Butyl-dimethyl-silanyloxy)-2-((R)-6-fluoro-chroman-2-yl)-ethyl]-[(R)-2-(tert-butyl-dimethyl-silanyloxy)-2-((S)-6-fluoro-chroman-2-yl)-ethyl]-amine
-
197706-50-6
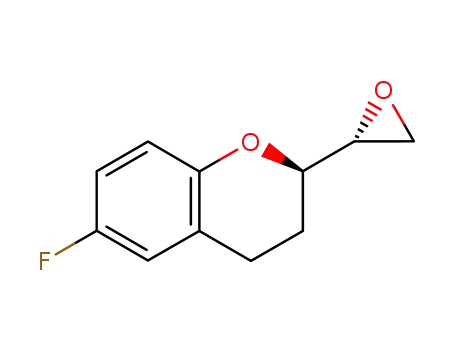
6-fluoro-(2R)-2-[(2R)-2-oxiranyl]-3,4-dihydrobenzopyran
-
303176-42-3
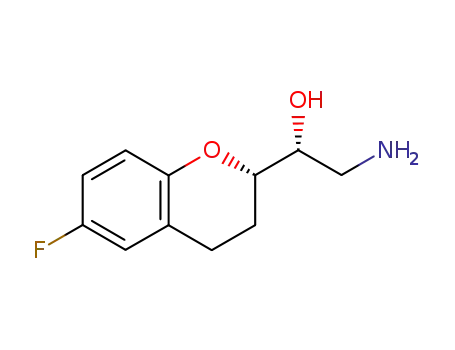
(R)-2-amino-1-[(S)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]ethanol
-
371-41-5
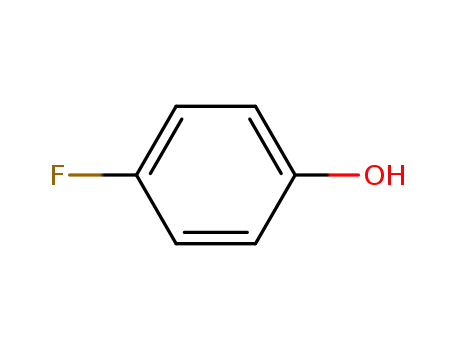
4-Fluorophenol
Relevant Products
-
Moxifloxacine Hcl
CAS:186826-86-8
-
Selexipag
CAS:475086-01-2
-
Azacitidine
CAS:320-67-2