475086-01-2
- Product Name:Selexipag
- Molecular Formula:C26H32N4O4S
- Purity:99%
- Molecular Weight:496.62168
Product Details:
CasNo: 475086-01-2
Molecular Formula: C26H32N4O4S
Buy High Grade Selexipag 475086-01-2,Export 475086-01-2 Efficient Transportation
- Molecular Formula:C26H32N4O4S
- Molecular Weight:496.62168
- PKA:3.82±0.40(Predicted)
- PSA:109.87000
- Density:1.210±0.06 g/cm3(Predicted)
- LogP:5.36970
475086-01-2 Relevant articles
Preparation method of medical intermediate
-
Paragraph 0027; 0029; 0030; 0032; 0033; 0035, (2021/10/11)
The invention provides a preparation met...
Synthesis method of diphenyl pyrazine derivative
-
Paragraph 0059-0063; 0064-0066; 0069-0070, (2021/06/13)
The invention discloses a synthesis meth...
Preparation method for Selexipag intermediate
-
, (2019/03/15)
The invention provides a method for prep...
METHOD FOR PREPARING PROSTACYCLIN RECEPTOR AGONIST
-
Paragraph 0076-079, (2018/03/01)
The present invention relates to prepara...
475086-01-2 Process route
-
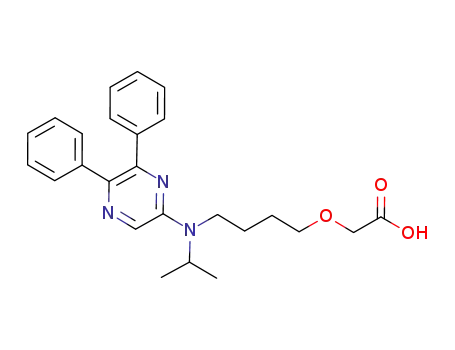
- 475085-57-5
MRE-269

-
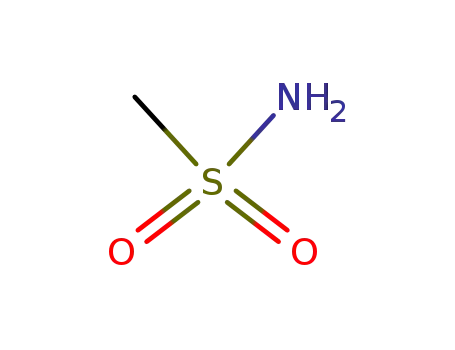
- 3144-09-0
methanesulfonamide

-
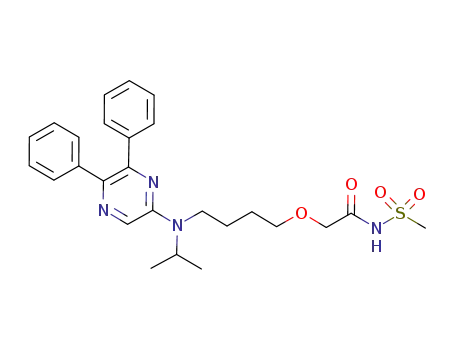
- 475086-01-2
Selexipag
| Conditions | Yield |
|---|---|
|
MRE-269; With 1,1'-carbonyldiimidazole; In dichloromethane; for 1h; Reflux;
methanesulfonamide; In dichloromethane; at 20 - 30 ℃; for 0.166667h;
With triethylamine; In dichloromethane; for 5h; Reagent/catalyst;
|
96.8% |
|
MRE-269; With oxalyl dichloride; In dichloromethane; at 5 - 20 ℃; for 2h;
methanesulfonamide; With triethylamine; In acetonitrile; at 10 ℃; for 5h; Reagent/catalyst; Solvent;
|
88% |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 65 - 70 ℃; for 2h;
methanesulfonamide; In tetrahydrofuran; for 0.5h;
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; for 0.666667h;
|
84.51% |
|
With 1,8-diazabicyclo[5.4.0]undec-7-ene; 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 20 ℃; for 12h;
|
77% |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; for 1h; Reflux;
methanesulfonamide; In tetrahydrofuran; for 0.166667h;
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; for 12h;
|
77% |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 20 ℃; for 1h; Reflux;
methanesulfonamide; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 20 ℃;
|
60.5% |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; for 1h; Reflux;
methanesulfonamide; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 0 - 5 ℃;
|
52% |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 20 ℃; for 1h; Heating / reflux;
methanesulfonamide; In tetrahydrofuran; at 20 ℃; for 0.166667h;
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 20 ℃;
|
|
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 65 - 70 ℃; for 0.75h;
methanesulfonamide; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 25 - 30 ℃; for 3h; Temperature; Solvent; Reagent/catalyst;
|
20 g |
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 20 ℃; for 1h; Inert atmosphere; Reflux;
methanesulfonamide; In tetrahydrofuran; at 20 ℃; for 0.166667h;
With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 20 ℃; Solvent; Reagent/catalyst;
|
272 mg |
|
MRE-269; methanesulfonamide; With dmap; In dichloromethane; at 20 - 30 ℃; for 0.25h;
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20 - 30 ℃; for 24h; Solvent; Reagent/catalyst; Temperature;
|
|
|
MRE-269; With 1,1'-carbonyldiimidazole; In tetrahydrofuran; at 60 - 65 ℃; for 1h;
methanesulfonamide; With 1,8-diazabicyclo[5.4.0]undec-7-ene; In tetrahydrofuran; at 0 - 65 ℃; for 12h;
|
-
![4-[N-(5,6-diphenylpyrazine-2-yl)-N-isopropylamino]-1-butanol](/upload/2024/1/b9247b01-a5e1-4383-839a-18341e6ee0fa.png)
- 475086-75-0
4-[N-(5,6-diphenylpyrazine-2-yl)-N-isopropylamino]-1-butanol

-
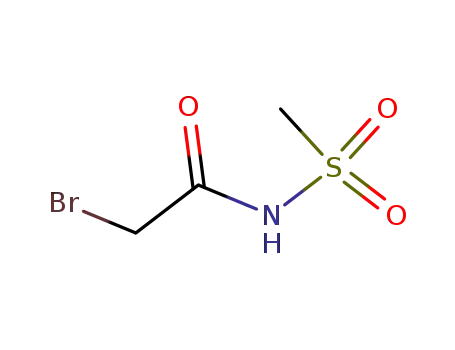
- 59504-75-5
N-(bromoacetyl)methanesulfonamide

-

- 475086-01-2
Selexipag
| Conditions | Yield |
|---|---|
|
With potassium tert-butylate; In 1,4-dioxane; at 25 - 30 ℃; for 3h; Temperature;
|
83.8% |
|
4-[N-(5,6-diphenylpyrazine-2-yl)-N-isopropylamino]-1-butanol; With potassium tert-butylate; In N,N-dimethyl-formamide; at -10 - 40 ℃; for 1h; Inert atmosphere;
N-(bromoacetyl)methanesulfonamide; In N,N-dimethyl-formamide; Temperature;
|
|
|
4-[N-(5,6-diphenylpyrazine-2-yl)-N-isopropylamino]-1-butanol; With potassium tert-butylate; In N,N-dimethyl-formamide; at 20 - 25 ℃; for 1h; Inert atmosphere;
N-(bromoacetyl)methanesulfonamide; In N,N-dimethyl-formamide; for 2h; Temperature; Inert atmosphere;
|
475086-01-2 Upstream products
-
475085-57-5

MRE-269
-
3144-09-0

methanesulfonamide
-
42042-71-7
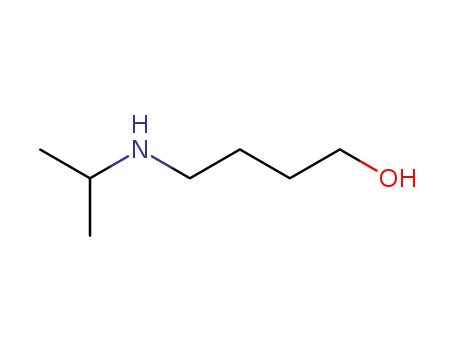
4-hydroxy-N-isopropylbutan-1-amine
-
475086-75-0
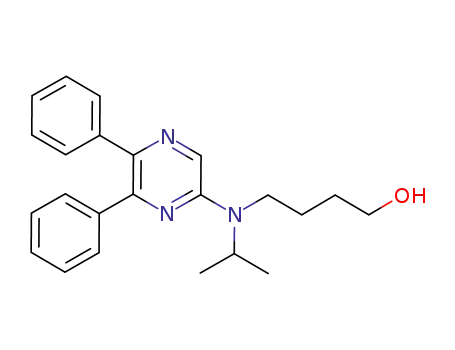
4-[N-(5,6-diphenylpyrazine-2-yl)-N-isopropylamino]-1-butanol
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Fosfomycin tromethamine
CAS:78964-85-9
-
Nebivolol hcl
CAS:152520-56-4