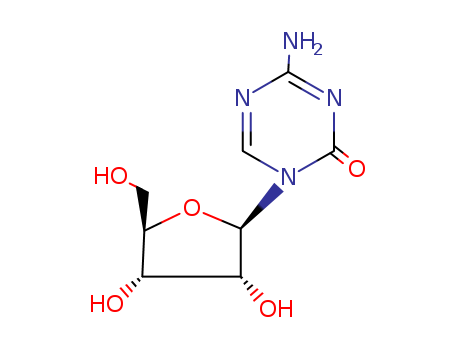
320-67-2
- Product Name:Azacitidine
- Molecular Formula:C8H12N4O5
- Purity:99%
- Molecular Weight:244.207
Product Details:
CasNo: 320-67-2
Molecular Formula: C8H12N4O5
Appearance: White-to-off-white crystalline solid
Chinese Factory Supply Top Purity 99% Azacitidine 320-67-2 Best Price
- Molecular Formula:C8H12N4O5
- Molecular Weight:244.207
- Appearance/Colour:White-to-off-white crystalline solid
- Vapor Pressure:1.18E-13mmHg at 25°C
- Melting Point:226-232 °C (dec.)(lit.)
- Refractive Index:1.823
- Boiling Point:534.5 °C at 760 mmHg
- PKA:13.46±0.70(Predicted)
- Flash Point:277 °C
- PSA:143.72000
- Density:2.08 g/cm3
- LogP:-2.58680
Azacitidine(Cas 320-67-2) Usage
|
Description |
Azacitidine, also known as 5-azacytidine and marketed under the name Vidaza, is an anti-cancer chemotherapy medication. It is a white crystalline solid or powder that is soluble in dimethyl sulfoxide and slightly soluble in various solvents. Classified as a demethylation and antimetabolite agent, Azacitidine acts by inhibiting DNA methyltransferase, functioning as a potent growth inhibitor and cytotoxic agent. It is indicated for the treatment of specific blood cell disorders and bone marrow cancers. |
|
Dosage |
For treatment of patients with myelodysplastic syndrome, the initial dose is 75mg/m2 administered intravenously or subcutaneously in daily doses for 7 days in 4-week intervals. The recommended maintenance dose may be increased to 100mg/m2 if there are no noteworthy effects after 2 treatment cycles and if the patient does not experience additional toxicity other than vomiting and nausea. Patients should undergo treatment that lasts for a minimum of 4 cycles. However, a partial or complete response may necessitate additional cycles other than the recommended 4 cycles. Treatment should not be discontinued if the patient is experiencing positive outcomes from the therapy. |
|
Elimination |
Intravenous administration of the radioactive form of the drug to cancer patients results in 85% elimination of Azacytidine through urinary excretion. Fecal excretion of the radioactive dose takes place in <1% of the administered drug in 3 days. The mean elimination of radioactivity through urine accounts for 50% of 14C-azacytidine administration. |
|
Originator |
Pharmion (US) |
|
Uses |
Azacitidine is administered subcutaneously for the treatment of myelodysplastic syndrome, with serum levels peaking within 30 minutes. It is excreted in urine. However, it is carcinogenic and teratogenic in rodents. The drug may cause leukopenia, thrombocytopenia, and neutropenia, leading to dose reduction or discontinuation. As a DNA methyltransferase inhibitor, Azacitidine incorporates into DNA, forming covalent adducts that deplete enzyme activity, inducing demethylation and reactivating silenced genes. It improves the efficiency of stem cell reprogramming. Incompatible with oxidizers, strong bases, acids, and various chemicals, Azacitidine is sensitive to light and oxidation, undergoing hydrolysis in aqueous buffers. It is used as an antineoplastic agent for the treatment of myelodysplastic syndromes, chronic myelomonocytic leukaemia, or acute myeloid leukaemia in individuals ineligible for stem cell transplants. Patients experiencing specific symptoms, such as persistent diarrhea, nausea interfering with eating, bloodstained urine, and other serious issues, should promptly contact their healthcare provider. |
|
Definition |
ChEBI: A N-glycosyl-1,3,5-triazine that is 4-amino-1,3,5-triazin-2(1H)-one substituted by a beta-D-ribofuranosyl residue via a N-glycosidic linkage. |
|
Brand name |
Vidaza (Pharmion). |
|
Therapeutic Function |
Antineoplastic |
|
Air & Water Reactions |
Slightly water soluble. Unstable in solution. |
|
Reactivity Profile |
5-Azacytidine is sensitive to light (may discolor). 5-Azacytidine is sensitive to oxidation. 5-Azacytidine is unstable in solution. 5-Azacytidine undergoes hydrolysis in aqueous buffers. 5-Azacytidine is incompatible with strong oxidizers. |
|
Fire Hazard |
Flash point data for 5-Azacytidine are not available; however, 5-Azacytidine is probably combustible. |
|
Safety Profile |
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic data. Poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by intravenous route: nausea, vomiting and dlarrhea, reduction in white cell count (luekopenia and agranulocytosis). An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin irritant. When heated to decomposition it emits toxic fumes of NOx. |
|
Synthesis |
The triazine ring of azacitidine is sensitive to water; this characteristic has made the synthesis of azacitidine a challenge, especially in manufacturing at commercial scale. A number of reports have appeared in order to avoid the use of water; however, these methods all have additional problems that render them undesirable for the large scale synthesis. A recent improved synthesis is depicted in the Scheme. 5- Azacytosine (1) was bis-silylated with HMDS in the presence of (NH4)SO4 to furnish trimethylsilylated azacytosine (2) in greater than 90% yield. Coupling of silylated azacytosine 2 with 1,2,3,5-tetra-O-acetyl-b-Dribofuranose (3) in DCM in the presence of TMS-triflate provided protected 5-azacitidine 4. The acetyl groups were then removed by using NaOMe in MeOH at rt. The crude azacitidine was crystallized from DMSO/MeOH to provide pure azacitidine (I). |
|
Drug interactions |
Potentially hazardous interactions with other drugs None known |
|
Carcinogenicity |
Azacitidine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals. |
|
Metabolism |
Azacitidine undergoes spontaneous hydrolysis and deamination mediated by cytidine deaminase. Following IV administration of radioactive azacitidine to 5 cancer patients, the cumulative urinary excretion was 85% of the radioactive dose. Faecal excretion accounted for <1% of administered radioactivity over three days. Mean excretion of radioactivity in urine following SC administration of [14C]-azacitidine was 50%. |
|
Waste Disposal |
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator. |
InChI:InChI=1/C8H12N4O5/c9-7-10-2-12(8(16)11-7)6-5(15)4(14)3(1-13)17-6/h2-6,13-15H,1H2,(H2,9,11,16)/t3-,4+,5+,6?/m1/s1
320-67-2 Relevant articles
An improved and scalable process for the synthesis of 5-azacytidine: An antineoplastic drug
Vujjini, Satish Kumar,Varanasi, Ganesh,Arevelli, Srinivas,Kandala, Sreenatha Charyulu,Tirumalaraju, Satyanarayana Raju,Bandichhor, Rakeshwar,Kagga, Mukkanti,Cherukupally, Praveen
, p. 303 - 306 (2013)
An improved, practical, and scalable pro...
Synthesis, Hydrolytic Stability, and Antileukemic Activity of Azacytidine Nucleoside Analogs
Bozhok,Kalinichenko,Kuz’mitskii,Golubeva
, p. 804 - 809 (2016)
New azacytidine nucleoside analogs with ...
Azacytidine methylate substance and preparation method, pharmaceutical composition and application thereof
-
Paragraph 0050-0052, (2020/01/14)
The invention discloses an azacytidine m...
Preparation method of azacitidine (by machine translation)
-
Paragraph 0024; 0027; 0028; 0031; 0032; 0035; 0036; 0039, (2019/10/01)
The invention belongs to the field, and ...
Method for preparing azacitidine by high-purity and low-calcination residue
-
Paragraph 0014; 0043; 0046-0048; 0051-0053; 0056-0058;, (2019/10/01)
The invention relates to the field of a ...
320-67-2 Process route
-
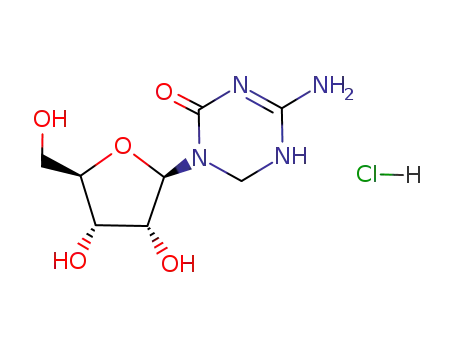
- 62402-31-7
5,6-dihydro-5-azacytidine hydrochloride

-
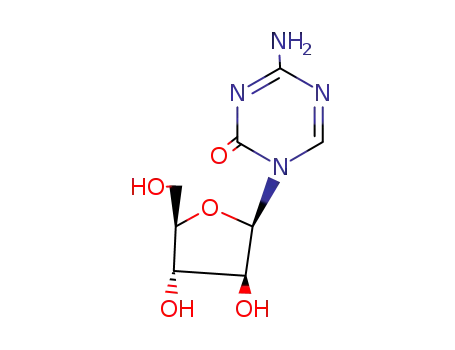
- 320-67-2,65886-71-7,114543-16-7
5-azacytosine arabinoside
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1: 83 percent / acetonitrile / 18 h / 40 - 50 °C
2: 83 percent / HCl / ethanol / 25 °C
3: 43 percent / 1 N NH4OH / 6 h / Heating
4: 1.) BSTFA, O2, 2.) Bu4NF / 1.) acetonitrile, 30 h, reflux, 2.) THF
With hydrogenchloride; ammonium hydroxide; tetrabutyl ammonium fluoride; oxygen; N,O-Bis(trimethylsilyl)trifluoroacetamide; In ethanol; acetonitrile;
|
|
|
Multi-step reaction with 5 steps
1: 83 percent / acetonitrile / 18 h / 40 - 50 °C
2: 67 percent / HCl / ethanol / 24 h / 4 °C
3: 83 percent / HCl / ethanol / 25 °C
4: 43 percent / 1 N NH4OH / 6 h / Heating
5: 1.) BSTFA, O2, 2.) Bu4NF / 1.) acetonitrile, 30 h, reflux, 2.) THF
With hydrogenchloride; ammonium hydroxide; tetrabutyl ammonium fluoride; oxygen; N,O-Bis(trimethylsilyl)trifluoroacetamide; In ethanol; acetonitrile;
|
|
|
Multi-step reaction with 3 steps
1: 36 percent / POCl3 / dimethylformamide / 15 h / 25 - 30 °C
2: 43 percent / 1 N NH4OH / 6 h / Heating
3: 1.) BSTFA, O2, 2.) Bu4NF / 1.) acetonitrile, 30 h, reflux, 2.) THF
With ammonium hydroxide; tetrabutyl ammonium fluoride; oxygen; N,O-Bis(trimethylsilyl)trifluoroacetamide; trichlorophosphate; In N,N-dimethyl-formamide;
|
-
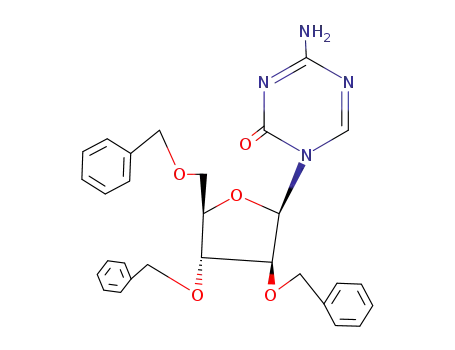
- 22432-98-0
1-(2,3,5-tri-O-benzyl-β-D-arabinofuranosyl)-5-azacytosine

-

- 320-67-2,65886-71-7,114543-16-7
5-azacytosine arabinoside

-
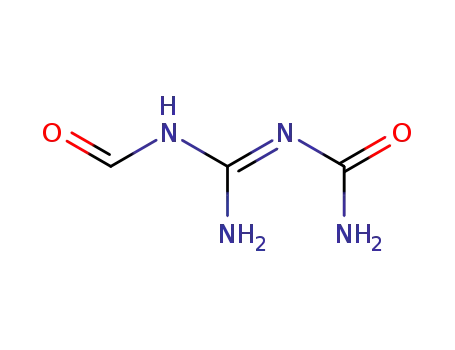
-
2-carbamoyl-1-formylguanidine
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; hydrogen; palladium on activated charcoal;
|
8% |
320-67-2 Upstream products
-
65126-88-7
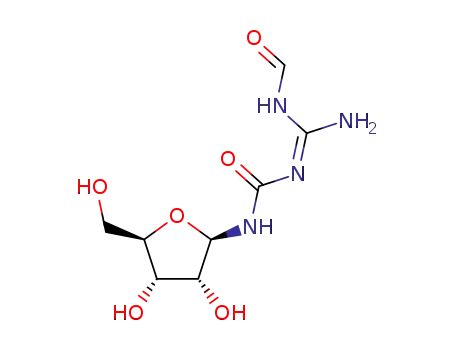
N-(Formylamidino)-N'-β-D-ribofuranosylharnstoff
-
13035-61-5
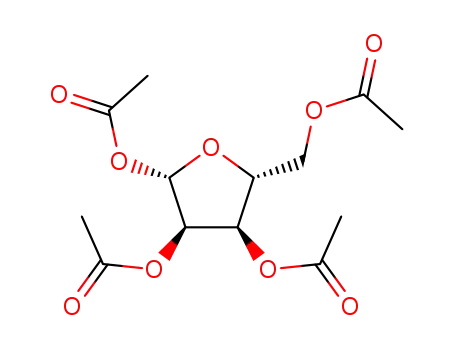
1,2,3,5-tetraacetylribose
-
52523-35-0
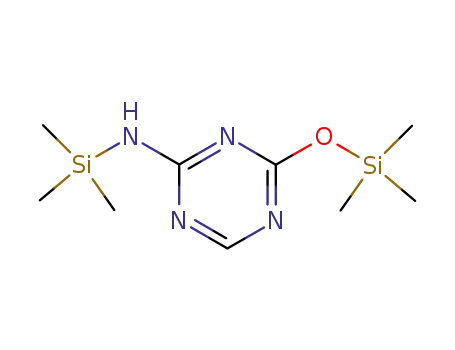
2-(trimethylsilylamino)-4-(trimethylsilyloxy)-s-triazine
-
10302-78-0
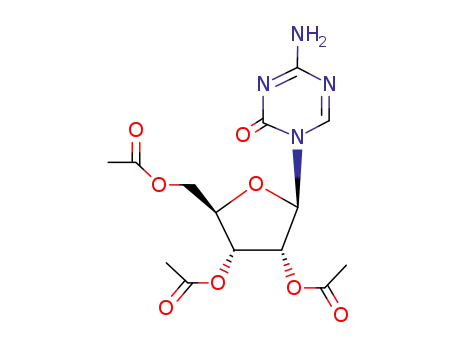
2,3,5-tri-O-acetyl-β-D-ribofuranose-4-amino-1,3,5-triazine
320-67-2 Downstream products
-
107036-45-3
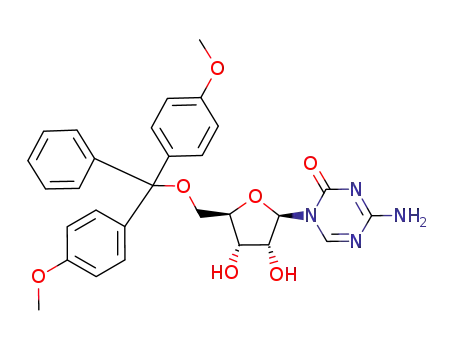
5'-O-(4,4'-dimethoxytrityl)-5-azacytidine
-
107036-47-5
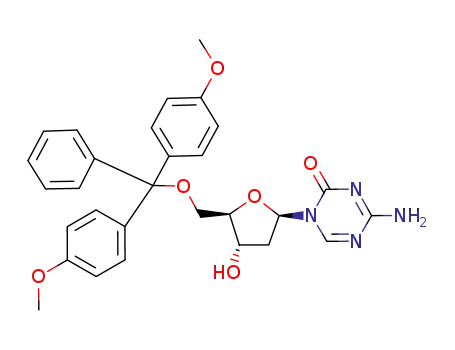
5'-O-(4,4'-dimethoxytrityl)-2'-deoxy-5-azacytidine
-
107036-48-6
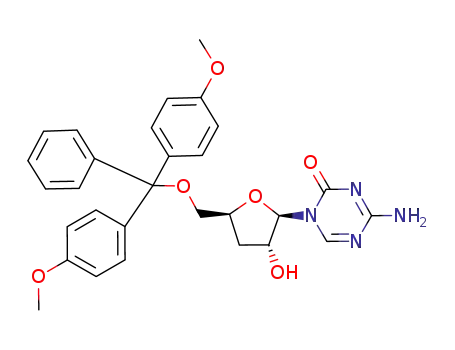
5'-O-(4,4'-dimethoxytrityl)-3'-deoxy-5-azacytidine
-
107036-46-4
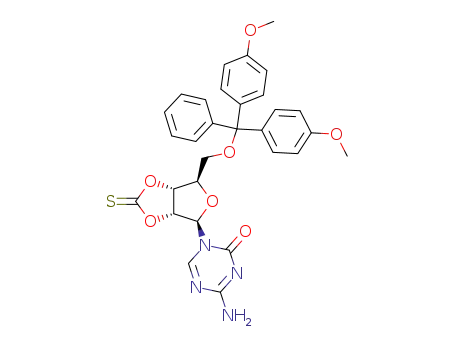
5'-O-(4,4'-dimethoxytrityl)-5-azacytidine 2',3'-O-(cyclic thiocarbonate)
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Nebivolol hcl
CAS:152520-56-4
-
Vericiguat
CAS:1350653-20-1