1269440-17-6
- Product Name:Encorafenib
- Molecular Formula:C22H27ClFN7O4S
- Purity:99%
- Molecular Weight:540.018
Product Details:
CasNo: 1269440-17-6
Molecular Formula: C22H27ClFN7O4S
Quality Manufacturer Supply Top Purity 99% Encorafenib 1269440-17-6 Competitive Price
- Molecular Formula:C22H27ClFN7O4S
- Molecular Weight:540.018
- PKA:5.94±0.10(Predicted)
- PSA:152.00000
- Density:1.45±0.1 g/cm3(Predicted)
- LogP:5.33960
Encorafenib(Cas 1269440-17-6) Usage
|
Overview |
Encorafenib is a medication sold under the brand name Braftovi, and it is used in the treatment of certain melanoma cancers. It belongs to the class of medications called kinase inhibitors and specifically acts as a small molecule BRAF inhibitor. |
| Chemical Structure | Encorafenib is a small molecule drug, but its detailed chemical structure is not provided in the information. The term "small molecule" indicates that it is a low molecular weight compound. |
| Mechanism of Action | Encorafenib functions as a BRAF inhibitor, targeting key enzymes in the MAPK (Mitogen-Activated Protein Kinase) signaling pathway. This pathway plays a crucial role in the regulation of cell growth and division. Aberrations in this pathway are often observed in various cancers, including melanoma and colorectal cancers. |
| Indications | Encorafenib is primarily used for the treatment of certain types of melanoma cancers. It is designed to inhibit the growth and division of melanoma cells by interfering with the signals within cancer cells that promote their continuous growth. |
| Combination Therapy | Encorafenib is sometimes used in combination with another chemotherapy drug called binimetinib (enco-bini). This combination is aimed not only at killing melanoma cells but also at preventing the development of new skin cancers. |
1269440-17-6 Relevant articles
COMPOUNDS AND COMPOSITIONS AS PROTEIN KINASE INHIBITORS
-
Page/Page column 58-59, (2011/04/14)
The invention provides a novel class of ...
Phase I Dose-Escalation and -Expansion Study of the BRAF Inhibitor Encorafenib (LGX818) in Metastatic BRAF-Mutant Melanoma
Jean-Pierre Delord; Caroline Robert; Marta Nyakas; Grant A. McArthur; Ragini Kudchakar; Amit Mahipal; Yasuhide Yamada; Ryan Sullivan; Ana Arance; Richard F. Kefford; Matteo S. Carlino; Manuel Hidalgo; Carlos Gomez-Roca; Daniela Michel; Abdelkader Seroutou; Vassilios Aslanis; Giordano Caponigro; Darrin D. Stuart; Laure Moutouh-de Parseval; Tim Demuth; Reinhard Dummer
CANCER THERAPY: CLINICAL, Volume 23, Issue 18 15 September 2017
Encorafenib monotherapy was then tested across a range of once-daily (50–700 mg) or twice-daily (75–150 mg) regimens in a phase I, open-label, dose-escalation and -expansion study in adult patients with histologically confirmed advanced/metastatic BRAF-mutant melanoma. Study objectives were to determine the maximum tolerated dose (MTD) and/or recommended phase II dose (RP2D), characterize the safety and tolerability and pharmacokinetic profile, and assess the preliminary antitumor activity of encorafenib.
1269440-17-6 Process route
-
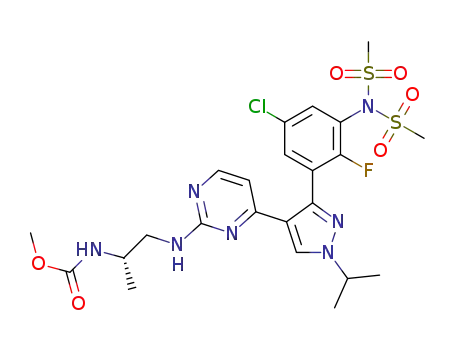
- 1269440-99-4
(S)-methyl 1-(4-(3-(5-chloro-2-fluoro-3-(N-(methylsulfonyl)methylsulfonamido)phenyl)-1-isopropyl-1H-pyrazol-4-yl)pyrimidin-2-ylamino)propan-2-ylcarbamate

-
![methyl N-[(2S)-1-({4-[3-(5-chloro-2-fluoro-3-methanesulfonamidophenyl)-1-(propan-2-yl)-1H-pyrazol-4-yl]pyrimidin-2-yl}amino)propan-2-yl]carbamate](/upload/2024/1/ed90f15a-f173-4845-83c4-7ef3c40c8ed0.png)
- 1269440-17-6
methyl N-[(2S)-1-({4-[3-(5-chloro-2-fluoro-3-methanesulfonamidophenyl)-1-(propan-2-yl)-1H-pyrazol-4-yl]pyrimidin-2-yl}amino)propan-2-yl]carbamate
| Conditions | Yield |
|---|---|
|
(S)-methyl 1-(4-(3-(5-chloro-2-fluoro-3-(N-(methylsulfonyl)methylsulfonamido)phenyl)-1-isopropyl-1H-pyrazol-4-yl)pyrimidin-2-ylamino)propan-2-ylcarbamate; With sodium hydroxide; In 2-methyltetrahydrofuran; water; at 20 - 23 ℃; for 0.5h;
With hydrogenchloride; In 2-methyltetrahydrofuran; water; pH=6 - 6.5;
With sodium hydrogencarbonate; In 2-methyltetrahydrofuran; water; pH=8.5;
|
-
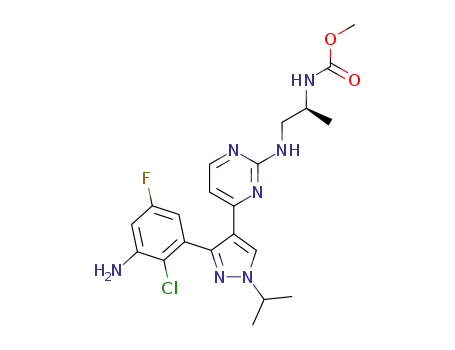
- 1269440-76-7
(S)-methyl 1-(4-(3-(3-amino-2-chloro-5-fluorophenyl)-1-isopropyl-1H-pyrazol-4-yl)pyrimidin-2-ylamino)propan-2-ylcarbamate

-
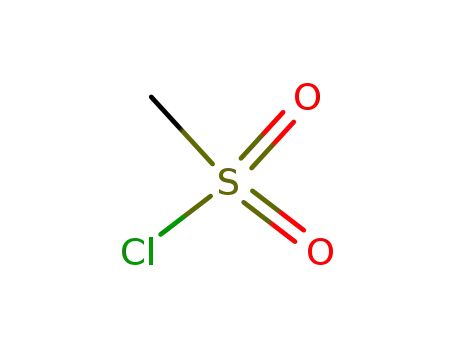
- 124-63-0
methanesulfonyl chloride

-
![methyl N-[(2S)-1-({4-[3-(5-chloro-2-fluoro-3-methanesulfonamidophenyl)-1-(propan-2-yl)-1H-pyrazol-4-yl]pyrimidin-2-yl}amino)propan-2-yl]carbamate](/upload/2024/1/ed90f15a-f173-4845-83c4-7ef3c40c8ed0.png)
- 1269440-17-6
methyl N-[(2S)-1-({4-[3-(5-chloro-2-fluoro-3-methanesulfonamidophenyl)-1-(propan-2-yl)-1H-pyrazol-4-yl]pyrimidin-2-yl}amino)propan-2-yl]carbamate
| Conditions | Yield |
|---|---|
|
With pyridine; In dichloromethane; at 20 ℃; for 16h;
|
1269440-17-6 Upstream products
-
1269440-76-7

(S)-methyl 1-(4-(3-(3-amino-2-chloro-5-fluorophenyl)-1-isopropyl-1H-pyrazol-4-yl)pyrimidin-2-ylamino)propan-2-ylcarbamate
-
124-63-0

methanesulfonyl chloride
-
1996-30-1
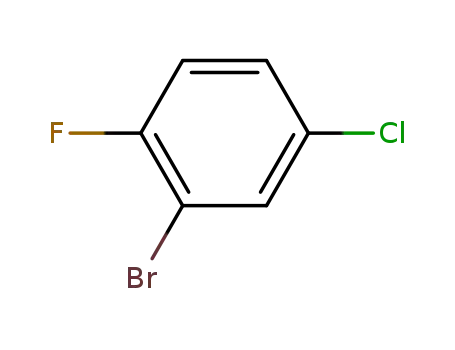
2-bromo-4-chloro-1-fluorobenzene
-
1269232-93-0
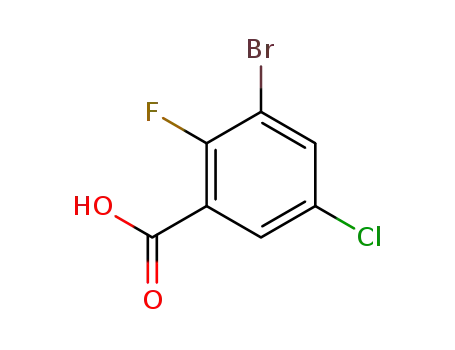
3-bromo-5-chloro-2-fluorobenzoic acid
Relevant Products
-
Lenacapavir
CAS:2189684-44-2
-
Daprodustat
CAS:960539-70-2
-
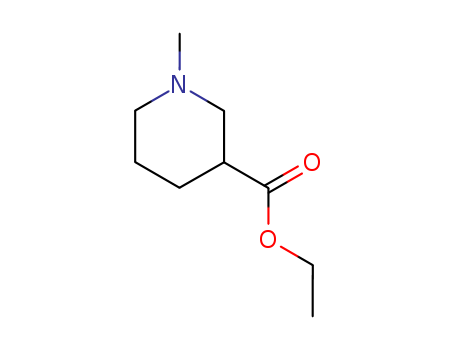
Ethyl 1-methylpiperidine-3-carboxylate
CAS:5166-67-6