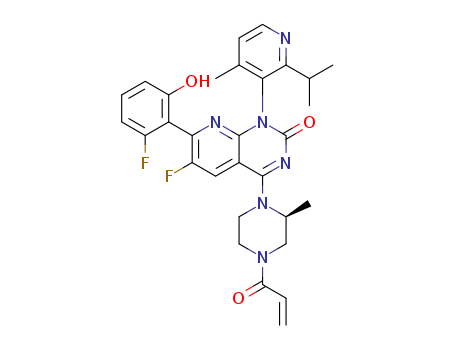
2296729-00-3
- Product Name:Sotorasib
- Molecular Formula:C30H30F2N6O3
- Purity:99%
- Molecular Weight:560.603
Product Details:
CasNo: 2296729-00-3
Molecular Formula: C30H30F2N6O3
Buy High Quality Sotorasib,Export 2296729-00-3 Best Price
- Molecular Formula:C30H30F2N6O3
- Molecular Weight:560.603
- Boiling Point:730.5±70.0 °C(Predicted)
- Density:1.36±0.1 g/cm3(Predicted)
Sotorasib (Cas 2296729-00-3) Usage
|
Description |
Sotorasib, sold under the brand names Lumakras and Lumykras, is an anti-cancer medication used to treat non-small-cell lung cancer (NSCLC). Specifically designed for adults who have undergone at least one prior treatment and have NSCLC that has either spread to other parts of the body or is unresectable, Sotorasib operates as a KRAS inhibitor. This means it targets the abnormal protein G12C mutation in the K-Ras gene (KRAS), which is implicated in various forms of cancer. |
|
Uses |
As a KRAS inhibitor, Sotorasib works by blocking the action of the mutated KRAS protein, disrupting its signaling pathway and thereby impeding the multiplication of cancer cells. Commonly known side effects of Sotorasib therapy, as noted by NCBI Bookshelf, include serum aminotransferase elevations and severe liver injury. Sotorasib's availability under the brand names Lumakras and Lumykras underscores its role in addressing refractory cases of non-small cell lung cancer, particularly those with the KRAS G12C mutation, which accounts for up to 13% of such cases. This small molecule inhibitor holds promise as an effective treatment strategy for individuals with advanced NSCLC who have exhausted other therapeutic options. |
2296729-00-3 Relevant articles
Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial
Adrianus Johannes de Langen, MD PhD Assist Prof Melissa L Johnson, MD Prof Julien Mazieres, MD PhD Prof Anne-Marie C Dingemans, MD PhD Giannis Mountzios, MD PhD Prof Miklos Pless, MD
, The Lancet, 2023
We randomly assigned (1:1) patients to oral sotorasib (960 mg once daily) or intravenous docetaxel (75 mg/m2 once every 3 weeks) in an open-label manner using interactive response technology. Randomisation was stratified by number of previous lines of therapy in advanced disease (1 vs 2 vs >2), ethnicity (Asian vs non-Asian), and history of CNS metastases (present or absent).
Discovery of a Covalent Inhibitor of KRASG12C (AMG 510) for the Treatment of Solid Tumors
Lanman, Brian A.,Allen, Jennifer R.,Allen, John G.,Amegadzie, Albert K.,Ashton, Kate S.,Booker, Shon K.,Chen, Jian Jeffrey,Chen, Ning,Frohn, Michael J.,Goodman, Guy,Kopecky, David J.,Liu, Longbin,Lopez, Patricia,Low, Jonathan D.,Ma, Vu,Minatti, Ana E.,Nguyen, Thomas T.,Nishimura, Nobuko,Pickrell, Alexander J.,Reed, Anthony B.,Shin, Youngsook,Siegmund, Aaron C.,Tamayo, Nuria A.,Tegley, Christopher M.,Walton, Mary C.,Wang, Hui-Ling,Wurz, Ryan P.,Xue, May,Yang, Kevin C.,Achanta, Pragathi,Bartberger, Michael D.,Canon, Jude,Hollis, L. Steven,McCarter, John D.,Mohr, Christopher,Rex, Karen,Saiki, Anne Y.,San Miguel, Tisha,Volak, Laurie P.,Wang, Kevin H.,Whittington, Douglas A.,Zech, Stephan G.,Lipford, J. Russell,Cee, Victor J.
, p. 52 - 65 (2020/01/09)
KRASG12C has emerged as a promising targ...
2296729-00-3 Process route
-
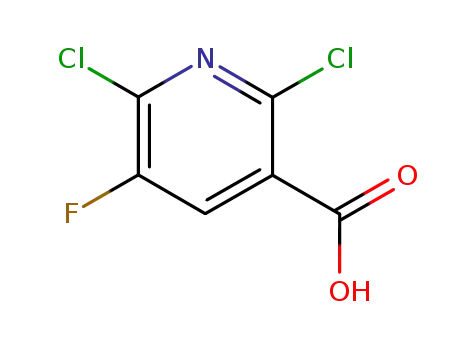
- 82671-06-5
2,6-dichloro-5-fluoro-3-pyridinecarboxylic acid

-
![(1R)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1H)-one](/upload/2024/1/a07ba3fd-6e2a-4baf-ace1-b1b7d5d56f78.png)
- 2252403-56-6,2296729-00-3
(1R)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1H)-one
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 7 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
1.2: 0.5 h / 0 °C
2.1: tetrahydrofuran; dichloromethane / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C / Cooling with ice
4.1: trichlorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 20 °C / Cooling with ice
6.1: potassium acetate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 / water; 1,4-dioxane / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
7.2: 0.17 h / 0 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: oxalyl dichloride / dichloromethane; N,N-dimethyl-formamide / 16 h / 20 °C
1.2: 0.5 h / 0 °C
2.1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 1 h / 75 °C / Cooling with ice
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C / Cooling with ice
4.1: trichlorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 20 °C / Cooling with ice
5.2: 0.08 h
6.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / water; dichloromethane; 1,4-dioxane / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
8.1: N-ethyl-N,N-diisopropylamine / dichloromethane / 0 °C
8.2: Chiralpak IC / 76507.7 Torr / Supercritical conditions
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 15 - 20 °C / Large scale
2.1: dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / toluene / 3.5 h / 0 - 45 °C / Large scale
4.2: 0.25 h / 25 °C / Large scale
5.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
6.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
6.2: 0.17 h / 0 °C
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 7 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 15 - 20 °C / Large scale
2.1: dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 20 °C
6.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
7.2: 0.17 h / 0 °C
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 15 - 20 °C / Large scale
2.1: dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / toluene / 3.5 h / 0 - 45 °C / Large scale
4.2: 0.25 h / 25 °C / Large scale
5.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
6.1: trifluoroacetic acid / dichloromethane / 20 °C / Large scale
6.2: Large scale
7.1: 1-methyl-pyrrolidin-2-one / 2 h / 20 - 30 °C
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; 1-methyl-pyrrolidin-2-one; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 8 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 15 - 20 °C / Large scale
2.1: dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 20 °C
6.1: potassium acetate; (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid / dichloromethane / 20 °C / Large scale
7.2: Large scale
8.1: 1-methyl-pyrrolidin-2-one / 2 h / 20 - 30 °C
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; 1-methyl-pyrrolidin-2-one; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 6 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
2.1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / toluene / 3 h / 0 - 45 °C / Large scale
4.2: 0.25 h / 25 °C / Large scale
5.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
6.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
6.2: 0.17 h / 0 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 7 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
2.1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / toluene / 3 h / 0 - 45 °C / Large scale
4.2: 0.25 h / 25 °C / Large scale
5.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
6.1: trifluoroacetic acid; potassium carbonate / dichloromethane; water; methanol / 14 h / 20 °C
7.1: 1-methyl-pyrrolidin-2-one / 2 h / 30 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; potassium carbonate; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; 1-methyl-pyrrolidin-2-one; methanol; dichloromethane; water; toluene;
|
|
|
Multi-step reaction with 7 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
2.1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 20 °C
6.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
7.2: 0.17 h / 0 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 8 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
2.1: oxalyl dichloride / dichloromethane; tetrahydrofuran / 1 h / 75 °C
2.2: 1 h / 0 °C
3.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C
4.1: N-ethyl-N,N-diisopropylamine; trichlorophosphate / acetonitrile / 1 h / 80 °C
5.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 20 °C
6.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
7.1: trifluoroacetic acid; potassium carbonate / dichloromethane; water; methanol / 14 h / 20 °C
8.1: 1-methyl-pyrrolidin-2-one / 2 h / 30 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; potassium carbonate; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; 1-methyl-pyrrolidin-2-one; methanol; dichloromethane; water; acetonitrile;
|
|
|
Multi-step reaction with 11 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / 15 - 20 °C / Large scale
1.2: 1.5 h / 0 °C / Large scale
2.1: dichloromethane / 4.25 h / 25 - 40 °C / Large scale
3.1: dichloromethane / 1 h / 0 - 23 °C / Large scale
4.1: sodium t-butanolate / 2-methyltetrahydrofuran / 3 h / 5 - 23 °C / Large scale
5.1: 2-methyltetrahydrofuran / 0.5 h / 75 °C / Inert atmosphere; Large scale
6.1: disodium hydrogenphosphate / water; tert-butyl methyl ether / 3 h / Large scale
7.1: trichlorophosphate; N-ethyl-N,N-diisopropylamine / toluene / 7 h / 30 °C / Large scale
8.1: N-ethyl-N,N-diisopropylamine / dichloromethane; toluene; Isopropyl acetate / 1 h / 20 °C / Large scale
9.1: potassium acetate; dichloro[bis(2-(diphenylphosphino)phenyl)ether]palladium(ll) / water; 2-methyltetrahydrofuran / 75 °C / Large scale
10.1: trifluoroacetic acid / dichloromethane / 4 h / 20 °C / Large scale
11.1: trifluoroacetic acid / 1-methyl-pyrrolidin-2-one / 1.5 h / 0 - 10 °C / Inert atmosphere; Large scale
With disodium hydrogenphosphate; oxalyl dichloride; dichloro[bis(2-(diphenylphosphino)phenyl)ether]palladium(ll); potassium acetate; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; sodium t-butanolate; trichlorophosphate; In 2-methyltetrahydrofuran; 1-methyl-pyrrolidin-2-one; dichloromethane; tert-butyl methyl ether; Isopropyl acetate; water; toluene;
|
|
|
Multi-step reaction with 8 steps
1.1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 20 °C
1.2: 0.5 h / 0 °C
2.1: dichloromethane; tetrahydrofuran / 1 h / 75 °C / Cooling with ice
3.1: oxalyl dichloride / tetrahydrofuran / 1 h / 0 °C
4.1: potassium hexamethylsilazane / tetrahydrofuran / 0.67 h / 20 °C / Cooling with ice
5.1: trichlorophosphate; N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 80 °C
6.1: N-ethyl-N,N-diisopropylamine / acetonitrile / 1 h / 20 °C / Cooling with ice
7.1: dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate / 1,4-dioxane; water / 1 h / 90 °C / Inert atmosphere
8.1: trifluoroacetic acid / dichloromethane / 1 h / 20 °C
8.2: 0.17 h / 0 °C
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; oxalyl dichloride; potassium acetate; potassium hexamethylsilazane; N-ethyl-N,N-diisopropylamine; N,N-dimethyl-formamide; trifluoroacetic acid; trichlorophosphate; In tetrahydrofuran; 1,4-dioxane; dichloromethane; water; acetonitrile;
|
-
![6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-4-[(2S)-2-methylpiperazin-1-yl]-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]pyrido[2,3-d]pyrimidin-2(1H)-one](/upload/2024/1/064c1d36-de1b-4df2-aa1b-3c3f60c544ad.png)
-
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-4-[(2S)-2-methylpiperazin-1-yl]-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]pyrido[2,3-d]pyrimidin-2(1H)-one

-
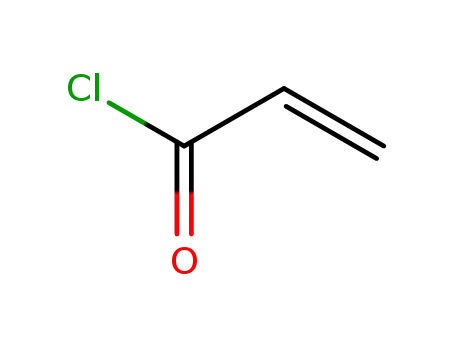
- 814-68-6,25189-84-8
acryloyl chloride

-
![(1R)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1H)-one](/upload/2024/1/a07ba3fd-6e2a-4baf-ace1-b1b7d5d56f78.png)
- 2252403-56-6,2296729-00-3
(1R)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-[4-methyl-2-(1-methylethyl)-3-pyridinyl]-4-[(2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl]pyrido[2,3-d]pyrimidin-2(1H)-one
| Conditions | Yield |
|---|---|
|
In 1-methyl-pyrrolidin-2-one; at 20 - 30 ℃; for 2h;
|
80% |
|
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-4-[(2S)-2-methylpiperazin-1-yl]-(1M)-1-[4-methyl-2-(propan-2-yl)pyridin-3-yl]pyrido[2,3-d]pyrimidin-2(1H)-one; acryloyl chloride; With N-ethyl-N,N-diisopropylamine; In dichloromethane; at 0 ℃;
In methanol; under 76507.7 Torr; Supercritical conditions;
|
2.25 g |
|
In 1-methyl-pyrrolidin-2-one; at 30 ℃; for 2h;
|
|
|
With trifluoroacetic acid; In 1-methyl-pyrrolidin-2-one; at 0 - 10 ℃; for 1.5h; Inert atmosphere; Large scale;
|
2296729-00-3 Upstream products
-
126325-50-6
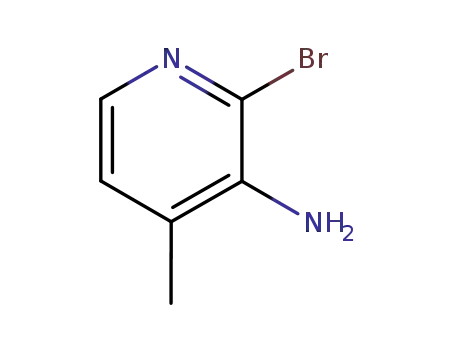
3-amino-2-bromo-4-methylpyridine
-
1698293-93-4
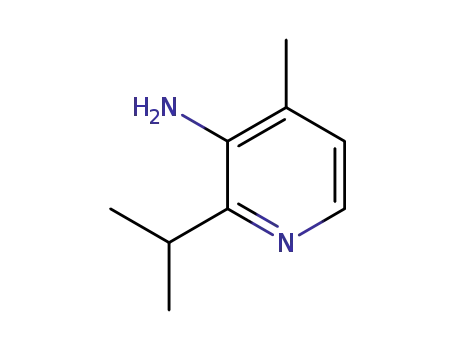
4-methyl-2-(1-methylethyl)-3-pyridinamine
-
82671-06-5

2,6-dichloro-5-fluoro-3-pyridinecarboxylic acid
-
113237-20-0
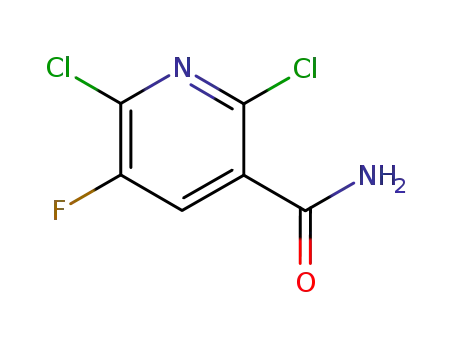
2,6-dichloro-5-fluoropyridine-3-carboxamide
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
N-Bromosuccinimide
CAS:128-08-5
-
Caspofungin Acetate
CAS:179463-17-3