56390-09-1
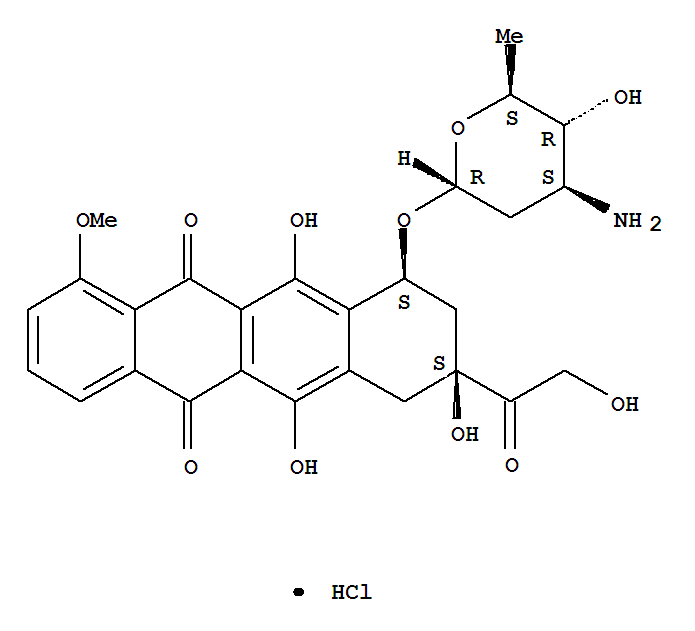
- Product Name:Epirubicin Hcl
- Molecular Formula:C27H29NO11.HCl
- Purity:99%
- Molecular Weight:579.99
Product Details:
CasNo: 56390-09-1
Molecular Formula: C27H29NO11.HCl
Appearance: Orange-red crystalline solid
Buy Quality Epirubicin Hcl ,Top Purity 56390-09-1 Lowest Price
- Molecular Formula:C27H29NO11.HCl
- Molecular Weight:579.99
- Appearance/Colour:Orange-red crystalline solid
- Vapor Pressure:9.64E-28mmHg at 25°C
- Melting Point:185 °C dec
- Refractive Index:1.709
- Boiling Point:810.3 °C at 760 mmHg
- Flash Point:443.8 °C
- PSA:206.07000
- Density:1.61 g/cm3
- LogP:1.50360
Epirubicin hydrochloride(Cas 56390-09-1) Usage
|
Description |
Epirubicin HCl is a type of chemotherapy medication that belongs to the anthracycline class of drugs. It is the hydrochloride salt of the 4'-epi-isomer of doxorubicin, another anthracycline antineoplastic antibiotic. Epirubicin works by inhibiting the replication of cancer cells, disrupting their ability to copy DNA and produce RNA and proteins necessary for their growth and survival. |
|
Chemical Properties |
Orange-Red Crystalline Solid |
|
Originator |
Erbamont (Italy) |
|
Uses |
Epirubicin hydrochloride is utilized in the treatment of various types of cancers, including: Breast cancer: Epirubicin injection is often used in combination with other cancer medicines to treat breast cancer in patients who have undergone surgery to remove the tumor. It helps to destroy any remaining cancer cells and reduce the risk of cancer recurrence. |
|
Brand name |
Ellence(Pfizer);FARMORUBICIN. |
InChI:InChI=1/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17+,22-,27-/m0/s1
56390-09-1 Relevant articles
Interaction of epirubicin HCl with surfactants: Effect of NaCl and glucose
N Erdi̇Nç,S Göktürk,M Tunçay
, Journal of Pharmaceutical Sciences 2010/5/17
The interaction of an antitumoural drug, Epirubicin HCl, with anionic (sodiumdodecylsulfate; SDS), cationic (cetyltrimethylammonium bromide; CTAB), and nonionic (t‐octylphenoxypolyethoxyethanol; TX‐100, polyoxyethylenesorbitanmonolaurate; Tween 20) surfactants has been studied by absorption spectra as a function of surfactant concentration ranging from the premicellar to postmicellar region.
Effects of Epigallocatechin Gallate on the Cytotoxicity of Epirubicin-HCl in Lung Cancer Cells
A Erdogan,A Ozkan
Letters in drug design & discovery
In this context, our study determined the most effective application sequence by evaluating the cytotoxic responses of epirubicin-HCl and EGCG according to the different application orders in A-549 cells (NSCLC). Methods: To demonstrate the apoptotic activity, we detected changes in mRNA ratios of Bax, a proapoptotic gene, and Bcl-2, an anti-apoptotic gene (Bax/Bcl2), as well as changes in the activity of caspase 3/7 enzyme.
Relevant Products
-
Moxifloxacine Hcl
CAS:186826-86-8
-
Irinotecan Hcl
CAS:100286-90-6
-
Sorafenib Tosylate
CAS:475207-59-1