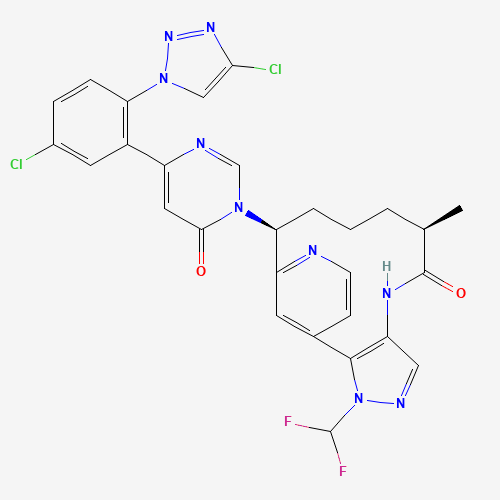
475207-59-1
- Product Name:Sorafenib Tosylate
- Molecular Formula:C21H16ClF3N4O3
- Purity:99%
- Molecular Weight:637.03
Product Details:
CasNo: 475207-59-1
Molecular Formula: C21H16ClF3N4O3
Appearance: White to off-white crystalline powder
Buy Reliable Quality Sorafenib Tosylate,Sale 475207-59-1 Lowest Price
- Molecular Formula:C21H16ClF3N4O3
- Molecular Weight:637.03
- Appearance/Colour:White to off-white crystalline powder
- Boiling Point:523.3 °C at 760 mmHg
- Flash Point:270.3 °C
- PSA:155.10000
- Density:1.454 g/cm3
- LogP:8.40910
Sorafenib Tosylate(Cas 475207-59-1) Usage
Sorafenib tosylate is an organosulfonate salt, consisting of sorafenib. This compound is a synthetic agent designed to target growth signaling and angiogenesis. Functioning as a multi-targeted kinase inhibitor, Sorafenib tosylate interferes with cancer cell growth by blocking proteins that signal cell division and inhibiting the formation of new blood vessels crucial for tumor growth. Unlike chemotherapy drugs, Sorafenib is not classified as such. Instead, it operates by disrupting specific cellular processes involved in cancer development, ultimately impeding tumor growth and potentially causing cancer cell death.
475207-59-1 Relevant articles
STUDY ON THE SCALEABLE SYNTHESIS OF SORAFENIB TOSYLATE FOR TREATMENT OF CANCER
MD Le, ND Vu, THA Pham, HT Cao
, Journal of Science and Technique, VOL. 13 NO. 03 (2018)
The objective of this paper is to study synthesis of sorafenib tosylate in large scale up to 220 gram. In this process, all intermediates, as well as final product, were recrystallized as purification method without chromatography, and it ample useful to reduce cost of commercial treatment cancer drug.
A Patient with Advanced Hepatocellular Carcinoma Treated with Sorafenib Tosylate Showed Massive Tumor Lysis with Avoidance of Tumor Lysis syndrome
Satoru Joshita, Kaname Yoshizawa, Kenji Sano, Satoshi Kobayashi, Tomohiro Sekiguchi, Susumu Morita, Atsushi Kamijo, Michiharu Komatsu, Takeji Umemura, Tetsuya Ichijo, Akihiro Matsumoto, Eiji Tanaka
, Internal Medicine, VOL 49 (2010)
Oral administration of sorafenib at 800 mg/day with careful observation was commenced in 2009. Laboratory investigations on day 7 showed massive tumor lysis. An abdominal CT showed multiple low density areas and tumor markers decreased, indicating extended tumor necrosis.
Relevant Products
-
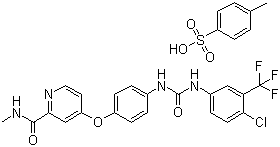
Milvexian
CAS:1802425-99-5
-
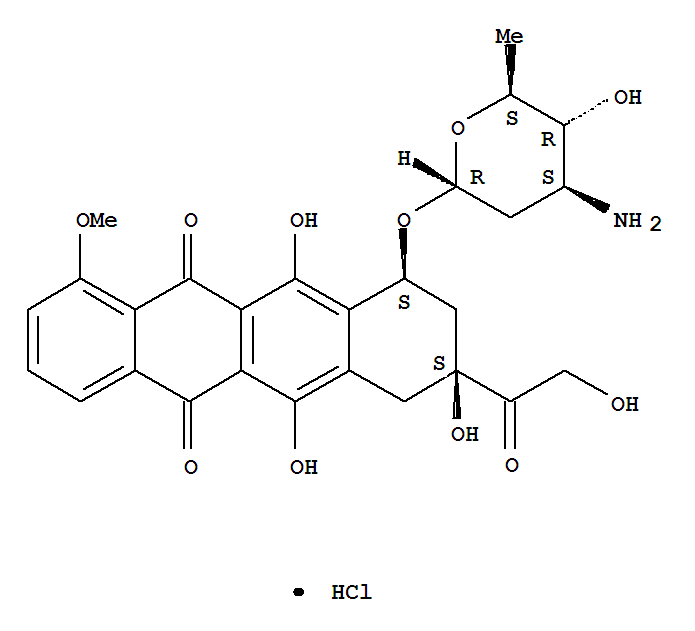
Epirubicin Hcl
CAS:56390-09-1
-
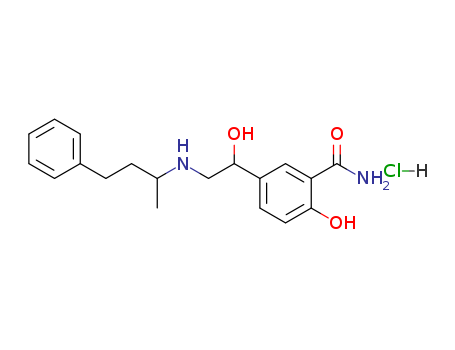
Labetalol hydrochloride
CAS:32780-64-6