1190389-15-1
- Product Name:Vibegron
- Molecular Formula:C26H28N4O3
- Purity:99%
- Molecular Weight:444.533
Product Details:
CasNo: 1190389-15-1
Molecular Formula: C26H28N4O3
Quality Manufacturer Supply Reliable Quality Vibegron 1190389-15-1 Reasonable Price
- Molecular Formula:C26H28N4O3
- Molecular Weight:444.533
- PKA:13.55±0.70(Predicted)
- PSA:96.25000
- Density:1.36±0.1 g/cm3(Predicted)
- LogP:3.16780
Vibegron(Cas 1190389-15-1) Usage
|
Description |
Vibegron is a selective beta-3 adrenergic receptor agonist. It belongs to the class of medications known as beta-3 adrenergic agonists, which work by targeting and activating beta-3 adrenergic receptors found in the bladder. This activation leads to relaxation of the bladder muscles, thereby increasing bladder capacity and reducing symptoms associated with OAB. |
| Uses |
Gemtesa (vibegron) is indicated for the treatment of adult patients with overactive bladder (OAB) who experience symptoms such as urge urinary incontinence, urgency, and urinary frequency. Overactive bladder is a condition characterized by a sudden and frequent urge to urinate, often leading to urinary incontinence (involuntary leakage of urine). Vibegron helps control these symptoms by relaxing the muscles of the bladder, allowing for better control over urination. |
1190389-15-1 Relevant articles
Synthesis of Vibegron Enabled by a Ketoreductase Rationally Designed for High pH Dynamic Kinetic Reduction
Xu, Feng,Kosjek, Birgit,Cabirol, Fabien L.,Chen, Haibin,Desmond, Richard,Park, Jeonghan,Gohel, Anupam P.,Collier, Steven J.,Smith, Derek J.,Liu, Zhuqing,Janey, Jacob M.,Chung, John Y. L.,Alvizo, Oscar
supporting information, p. 6863 - 6867 (2018/05/08)
Described here is an efficient stereosel...
The beta-3 adrenergic receptor agonist vibegron for the treatment of overactive bladder
Mi Wei, Luo Xi, Wei Bo
Modern medicine and clinical, 2017
Overactive bladder has become a major disease that troubles people. In recent years, a highly selective β_3 adrenergic receptor agonist has made great progress as a potential treatment for overactive bladder. vibegron is a Urinary incontinence treatment drugs with novel mechanisms of action have changed the idea of using traditional anticholinergic drugs to treat urinary incontinence for more than 30 years, thereby improving bladder filling and urine storage capacity. Mainly from the drug overview, related background, synthesis route, pharmacological effects, and clinical Research, safety and other aspects will be introduced.
PROCESS FOR MAKING BETA 3 AGONISTS AND INTERMEDIATES
-
Page/Page column 24-25, (2013/05/21)
The present invention is directed to pro...
1190389-15-1 Process route
-
![tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate](/upload/2024/1/963b073b-d020-4178-b0b5-42d56cf91770.png)
- 1190393-25-9
tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate

-
![(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide](/upload/2024/1/2ade2b13-5bcc-4db0-807d-5c2a43ad33aa.png)
- 1190389-15-1
(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide
| Conditions | Yield |
|---|---|
|
tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate; With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1.5h;
With sodium hydrogencarbonate; pH=8 - 9;
|
60% |
|
tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate; With trifluoroacetic acid; In dichloromethane; at 20 ℃; for 1.5h;
With sodium hydrogencarbonate; pH=8 - 9;
|
60% |
-
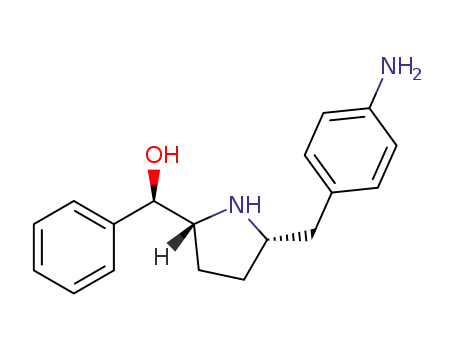
- 1295539-30-8
(R)-((2R,5S)-5-(4-aminobenzyl)pyrrolidin-2-yl)(phenyl)methanol

-
-
C8H7N2O3(1-)*Na(1+)

-
![(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide](/upload/2024/1/2ade2b13-5bcc-4db0-807d-5c2a43ad33aa.png)
- 1190389-15-1
(6S)-N-[4-({(2S,5R)-5-[(R)-hydroxy(phenyl)methyl]pyrrolidin-2-yl}methyl)phenyl]-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-a]pyrimidine-6-carboxamide
| Conditions | Yield |
|---|---|
|
With pyridine; hydrogenchloride; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In water; isopropyl alcohol; at 5 - 15 ℃; for 1h; pH=3.1 - 3.7; Inert atmosphere;
|
93% |
|
(R)-((2R,5S)-5-(4-aminobenzyl)pyrrolidin-2-yl)(phenyl)methanol; C8H7N2O3(1-)*Na(1+); With hydrogenchloride; In water; isopropyl alcohol; at 35 ℃; for 0.333333h; pH=3.3 - 3.5; Inert atmosphere;
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In water; isopropyl alcohol; at 20 ℃;
With ammonium hydroxide; In water; isopropyl alcohol; pH=Ca. 8.6;
|
14.3 g |
|
With hydrogenchloride; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In water; isopropyl alcohol; at 20 - 35 ℃; Concentration; Reagent/catalyst; pH-value; Temperature; Inert atmosphere;
|
14.3 g |
|
With hydrogenchloride; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In water; isopropyl alcohol; at 35 ℃; pH=3.3 - 3.5;
|
14.3 g |
1190389-15-1 Upstream products
-
1190393-25-9
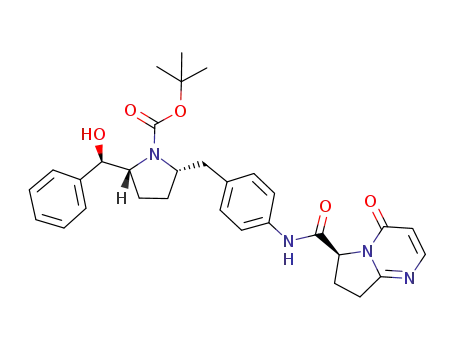
tert-butyl(2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-[4-({[(6S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]pyrimidin-6-yl]carbonyl}amino)benzyl]pyrrolidine-1-carboxylate
-
1190391-90-2
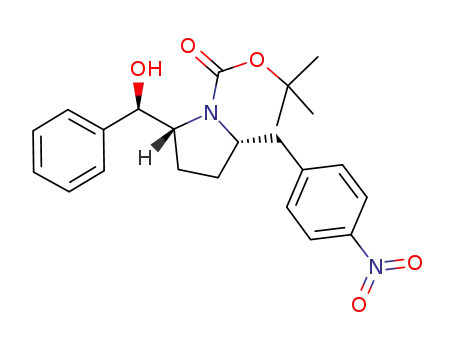
tert-butyl (2R,5S)-2-[(R)-hydroxy(phenyl)methyl]-5-(4-nitrobenzyl)pyrrolidine-1-carboxylate
-
1190392-23-4
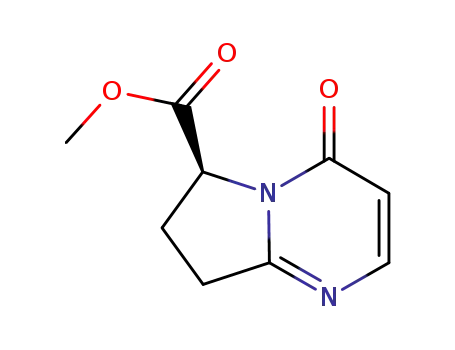
methyl [6(S)-4-oxo-4,6,7,8-tetrahydropyrrolo[1,2-α]]pyrimidine-6-carboxylate
-
173142-47-7
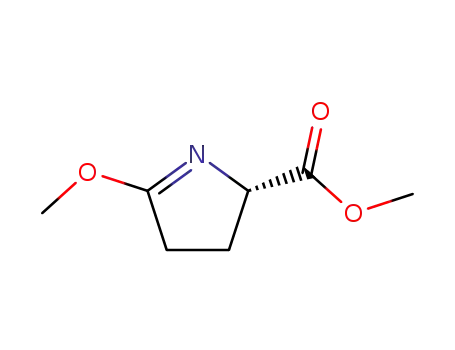
methyl (2S)-5-methoxy-3,4-dihydro-2H-pyrrole-2-carboxylate
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Midostaurin
CAS:120685-11-2
-
Acalabrutinib
CAS:1420477-60-6