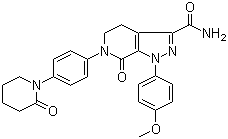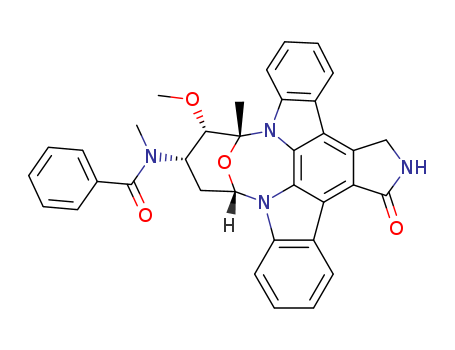
120685-11-2
- Product Name:Midostaurin
- Molecular Formula:C35H30 N4 O4
- Purity:99%
- Molecular Weight:570.648
Product Details:
CasNo: 120685-11-2
Molecular Formula: C35H30 N4 O4
Buy High Quality Midostaurin,Best Quality 120685-11-2 Customized Supply
- Molecular Formula:C35H30 N4 O4
- Molecular Weight:570.648
- Melting Point:235-260℃
- Boiling Point:°Cat760mmHg
- PKA:14.19±0.70(Predicted)
- Flash Point:°C
- PSA:77.73000
- Density:1.46g/cm3
- LogP:6.23560
Midostaurin 120685-11-2 Usage
|
Description |
Midostaurin, available under the brand names Rydapt and Tauritmo by Novartis, is a multi-targeted protein kinase inhibitor designed for the treatment of various conditions, including acute myeloid leukemia (AML), myelodysplastic syndrome, and advanced systemic mastocytosis. It is part of a combination therapy for AML, where it is used alongside daunorubicin and cytarabine, both chemotherapy drugs. |
|
Uses |
Midostaurin, pronounced MY-doh-STAW-rin, belongs to the class of medications known as kinase inhibitors. It is utilized as an antineoplastic agent to address high-risk AML with specific mutations, as well as aggressive systemic mastocytosis (ASM). In April 2017, the FDA approved midostaurin for the treatment of adult patients with newly diagnosed, FLT3 mutation-positive AML. This approval was granted for use in combination with standard cytarabine and daunorubicin induction, along with cytarabine consolidation. Additionally, midostaurin is indicated for advanced systemic mastocytosis, systemic mastocytosis with associated haematological neoplasm, and mast cell leukaemia. |
120685-11-2 Relevant articles
Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation
List of authors. Richard M. Stone, M.D., Sumithra J. Mandrekar, Ph.D., Ben L. Sanford, M.S., Kristina Laumann, B.A., Susan Geyer, Ph.D., Clara D. Bloomfield, M.D., Christian Thiede, M.D., Thomas W. Prior, Ph.D., Konstanze Döhner, M.D., Guido Marcucci, M.D., Francesco Lo-Coco, M.D., Rebecca B. Klisovic, M.D
N Engl J Med 2017; 377:454-464
Patients were randomly assigned to receive standard chemotherapy (induction therapy with daunorubicin and cytarabine and consolidation therapy with high-dose cytarabine) plus either midostaurin or placebo; those who were in remission after consolidation therapy entered a maintenance phase in which they received either midostaurin or placebo.
Efficacy and Safety of Midostaurin in Advanced Systemic Mastocytosis
Jason Gotlib, M.D., Hanneke C. Kluin-Nelemans, M.D., Ph.D., Tracy I. George, M.D., Cem Akin, M.D., Ph.D., Karl Sotlar, M.D., Olivier Hermine, M.D., Ph.D., Farrukh T. Awan, M.D., Elizabeth Hexner, M.D., Michael J. Mauro, M.D., David W. Sternberg, M.D., Ph.D., Matthieu Villeneuve, M.Sc., Alice Huntsman Labed, Ph.D
N Engl J Med 2016; 374:2530-2541
We conducted an open-label study of oral midostaurin at a dose of 100 mg twice daily in 116 patients, of whom 89 with mastocytosis-related organ damage were eligible for inclusion in the primary efficacy population; 16 had aggressive systemic mastocytosis, 57 had systemic mastocytosis with an associated hematologic neoplasm, and 16 had mast-cell leukemia.
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Ixazomib citrate
CAS:1201902-80-8
-
Vibegron
CAS:1190389-15-1