1420477-60-6
- Product Name:Acalabrutinib
- Molecular Formula:C26H23N7O2
- Purity:99%
- Molecular Weight:465.514
Product Details:
CasNo: 1420477-60-6
Molecular Formula: C26H23N7O2
Buy High Grade 1420477-60-6 Fast Shipping, Export Acalabrutinib
- Molecular Formula:C26H23N7O2
- Molecular Weight:465.514
- PKA:11.47±0.70(Predicted)
- PSA:118.51000
- Density:1.37±0.1 g/cm3(Predicted)
- LogP:3.90470
Acalabrutinib(Cas 1420477-60-6) Usage
|
Description |
Acalabrutinib, marketed as Calquence, is a medication utilized for the treatment of various non-Hodgkin lymphomas, specifically mantle cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma. This tyrosine kinase inhibitor (TKI) is employed in both relapsed and treatment-naive scenarios. Calquence (acalabrutinib) is a drug used to treat patients with blood cancers, including chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL) that does not respond to other treatments or returns following initial treatment response. |
| Uses | Acalabrutinib, classified as a chemotherapy and antineoplastic drug, operates by inhibiting the enzyme tyrosine kinase, impeding cell growth and division. In clinical studies like ASCEND and ELEVATE, acalabrutinib monotherapy and its combination with obinutuzumab exhibited enhanced efficacy and a well-tolerated safety profile, establishing its effectiveness and safety in the treatment of chronic lymphocytic leukemia. Acalabrutinib works by blocking Bruton's tyrosine kinase (BTK) signaling. This helps stop cancerous B cells from surviving and multiplying, which may slow the spread of cancer. This trial also checks for MRD after treatment with obinutuzumab and venetoclax. On November 21, 2019, the U.S. Food and Drug Administration (FDA) announced it has approved the use of acalabrutinib (CALQUENCE, AstraZeneca) as a monotherapy for the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). |
1420477-60-6 Relevant articles
Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial
Prof Michael Wang, MDa,b miwang@mdanderson.org ∙ Prof Simon Rule, MDc ∙ Prof Pier Luigi Zinzani, MDd ∙ Prof Andre Goy, MDe ∙ Prof Olivier Casasnovas, MDf ∙ Stephen D Smith, MDg
, The Lancet, Volume 391, Issue 10121p659-667February 17, 2018
In this open-label, phase 2 study, oral acalabrutinib (100 mg twice per day) was given to patients with relapsed or refractory mantle cell lymphoma, until disease progression or unacceptable toxicity. The primary endpoint was overall response assessed according to the Lugano classification, and safety analyses were done in all participants.
Resistance to Acalabrutinib in CLL Is Mediated Primarily By BTK Mutations
Jennifer Woyach MD 1, Ying Huang MS, MA * 2, Kerry Rogers * 2, Seema A. Bhat MD 2, Michael R. Grever MD 2, Arletta Lozanski * 2, Tzyy-Jye Doong * 2, James S. Blachly MD 2, Gerard Lozanski MD 2, Dan Jones MD PhD 2, John C. Byrd MD 3
, Blood, Volume 134, Supplement 1, 13 November 2019, Page 504
All patients (pts) treated at The Ohio State University and enrolled on an IRB approved phase 1b/2 study in CLL were included in this analysis. Beginning 12 months (mos) after the initiation of Acalabrutinib, pts underwent deep sequencing every 3-6 cycles using a digital droplet PCR assay for BTK C481S or Ion Torrent Sequencing for any BTK or PLCG2 mutations.
1420477-60-6 Process route
-
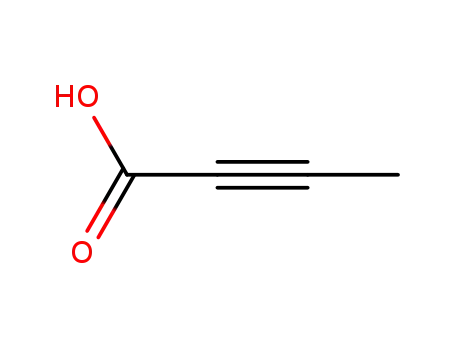
- 590-93-2
2-Butynoic acid

-
![4-{8-amino-3-[(2S)-pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide](/upload/2024/1/b91e5cf1-0cdb-49aa-b8ed-eabe5fc89d03.png)
- 1420478-90-5
4-{8-amino-3-[(2S)-pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide

-
![4-{8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide](/upload/2024/1/87a68740-e154-4e1c-8b32-22ab38155f88.png)
- 1420477-60-6,1952316-43-6
4-{8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide
| Conditions | Yield |
|---|---|
|
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In dichloromethane; at 20 - 30 ℃; for 3h;
|
90% |
|
With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine; In dichloromethane; Large scale;
|
74% |
|
With 1-hydroxy-pyrrolidine-2,5-dione; dicyclohexyl-carbodiimide; In dichloromethane; at -15 - 5 ℃; for 3h; Reagent/catalyst;
|
72% |
|
With triethylamine; HATU; In dichloromethane; at 20 ℃; for 0.5h;
|
18% |
|
With triethylamine; HATU; In dichloromethane; at 20 ℃; for 0.5h;
|
18% |
|
With triethylamine; HATU; In dichloromethane; at 20 ℃; for 0.5h;
|
18% |
|
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; at 20 ℃; Reagent/catalyst;
|
219 g |
|
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In dichloromethane; at 20 ℃; for 2h;
|
9.2 g |
|
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate; In dichloromethane; at 20 ℃; for 2h;
|
9.2 g |
|
2-Butynoic acid; With 1H-imidazole; In dichloromethane; at 0 - 30 ℃; for 0.666667h;
With pivaloyl chloride; In dichloromethane; at 0 - 0.1 ℃; for 1.08333h;
4-{8-amino-3-[(2S)-pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide; In dichloromethane; for 0.5h;
|
-
![4-[8-chloro-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridylbenzamide](/upload/2024/1/68002a66-5919-402b-bf92-0bd1309fe68a.png)
-
4-[8-chloro-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl]-N-2-pyridylbenzamide

-
![4-{8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide](/upload/2024/1/87a68740-e154-4e1c-8b32-22ab38155f88.png)
- 1420477-60-6,1952316-43-6
4-{8-amino-3-[(2S)-1-(1-oxo-2-butyn-1-yl)pyrrolidin-2-yl]imidazo[1,5-a]pyrazin-1-yl}-N-(pyridin-2-yl)benzamide
| Conditions | Yield |
|---|---|
|
With ammonia; In isopropyl alcohol; at 0 - 120 ℃; for 25h; Temperature; Solvent; Autoclave;
|
88% |
|
With ammonia; In isopropyl alcohol; at 0 - 120 ℃;
|
86% |
1420477-60-6 Upstream products
-
1418307-17-1
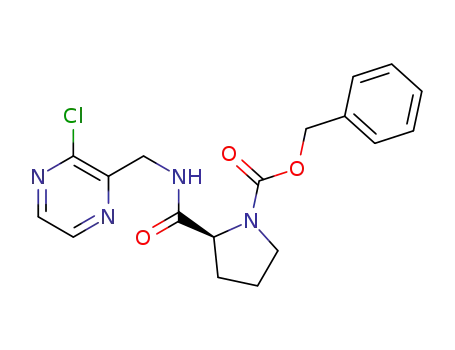
benzyl (S)-2-(((3-chloropyrazin-2-yl)methyl)carbamoyl)pyrrolidine-1-carboxylate
-
1418307-18-2
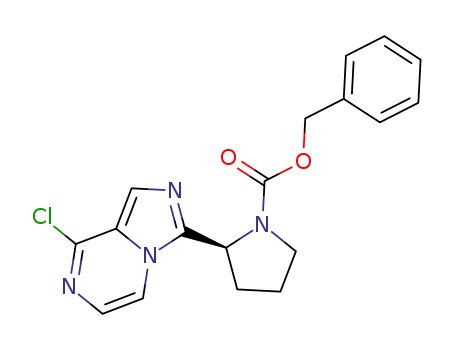
(S)-2-(8-chloroimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester
-
1420478-87-0
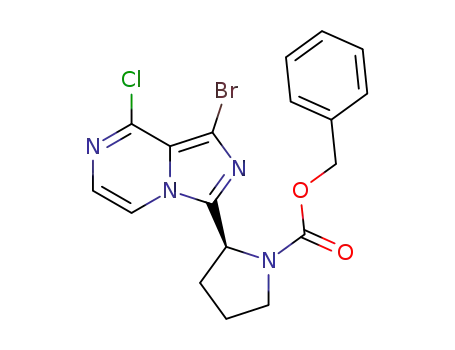
benzyl (2S)-2-(1-bromo-8-chloro-imidazo[1,5-a]pyrazin-3-yl)pyrrolidine-1-carboxylate
-
1420478-88-1
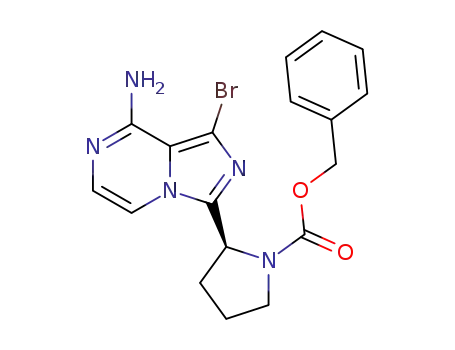
benzyl (2S)-2-(8-amino-1-bromo-imidazo[1,5-a]pyrazin-3-yl)pyrrolidine-1-carboxylate
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Vibegron
CAS:1190389-15-1
-
Elacestrant dihydrochloride
CAS:1349723-93-8