763113-22-0
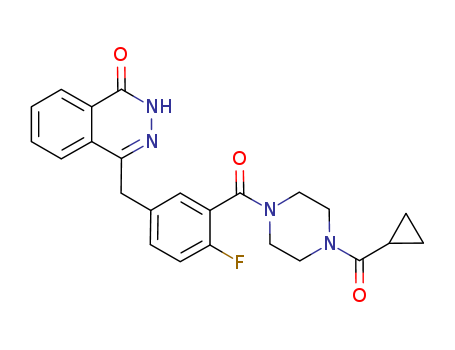
- Product Name:Olaparib
- Molecular Formula:C24H23FN4O3
- Purity:99%
- Molecular Weight:434.47
Product Details:
CasNo: 763113-22-0
Molecular Formula: C24H23FN4O3
Factory Sells 763113-22-0 Safe Shipping, Buy High Grade Olaparib
- Molecular Formula:C24H23FN4O3
- Molecular Weight:434.47
- Refractive Index:1.702
- PKA:12.07±0.40(Predicted)
- PSA:86.37000
- Density:1.43 g/cm3
- LogP:2.22320
Olaparib(Cas 763113-22-0) Usage
|
Description |
Olaparib, marketed as Lynparza, is an oral medication developed by AstraZeneca. It falls under the class of poly(ADP-ribose) polymerase (PARP) inhibitors, designed to specifically target cancer cells with certain genetic mutations. |
|
Chemical Properties |
White Solid |
|
Uses |
Breast Cancer Treatment: Olaparib is approved for HER2-negative breast cancer patients with specific germline mutations in the BRCA1 or BRCA2 genes. High-Risk Early-Stage Breast Cancer: It's used post-surgery in adults with high-risk early-stage breast cancer that has been treated with chemotherapy before or after surgery. |
|
Definition |
ChEBI: A member of the class of N-acylpiperazines obtained by formal condensation of the carboxy group of 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazin-1-yl)methyl]benzoic acid with the free amino group of N-(cyclpropylcarbonyl)pi erazine; used to treat advanced ovarian cancer. |
| Onset of Action | The time it takes for olaparib to start working varies depending on the type of cancer being treated. It generally ranges from two to five months, with ovarian cancer treatment potentially taking at least three months to show effectiveness. |
InChI:InChI=1/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
763113-22-0 Relevant articles
Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation
List of authors. Mark Robson, M.D., Seock-Ah Im, M.D., Ph.D., Elżbieta Senkus, M.D., Ph.D., Binghe Xu, M.D., Ph.D., Susan M. Domchek, M.D., Norikazu Masuda, M.D., Ph.D., Suzette Delaloge, M.D., Wei Li, M.D., Nadine Tung, M.D., Anne Armstrong, M.D., Ph.D., Wenting Wu, Ph.D., Carsten Goessl, M.D
, N Engl J Med 2017; 377:523-533
Of the 302 patients who underwent randomization, 205 were assigned to receive olaparib and 97 were assigned to receive standard therapy. Median progression-free survival was significantly longer in the olaparib group than in the standard-therapy group (7.0 months vs. 4.2 months; hazard ratio for disease progression or death, 0.58; 95% confidence interval, 0.43 to 0.80; P<0.001).
PARP inhibitor olaparib is safe and effective in patients with BRCA1 and BRCA2 mutations
Lisa Hutchinson
, Nature Reviews Clinical Oncology volume 7, page549 (2010)
In the first cohort, patients received 400 mg olaparib twice daily, and in the second cohort they received 100 mg olaparib twice daily. The primary end point was objective response rate (ORR) and safety and tolerability were also assessed.
763113-22-0 Upstream products
-
763114-26-7
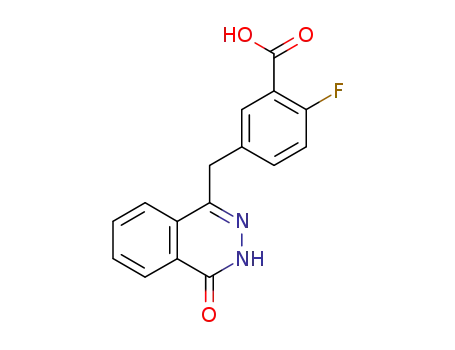
2-fluoro-5-[(4’-oxo-3’H-phthalazin-1’-yl)methyl]benzoic acid
-
709-45-5
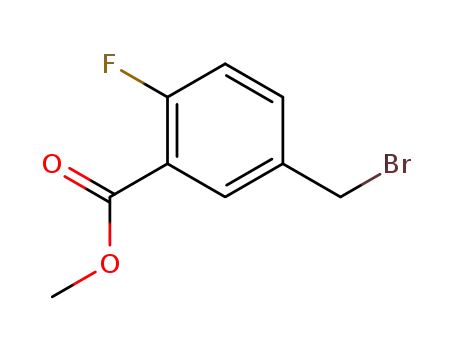
2-fluoro-5-bromomethyl benzoic acid methyl ester
-
108-86-1
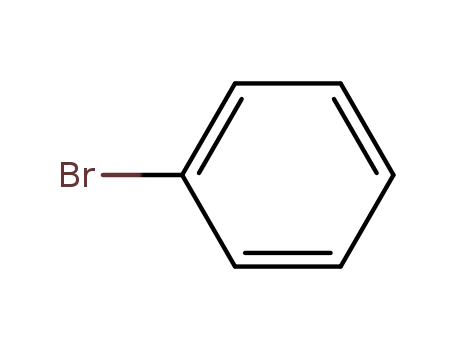
bromobenzene
-
218301-22-5
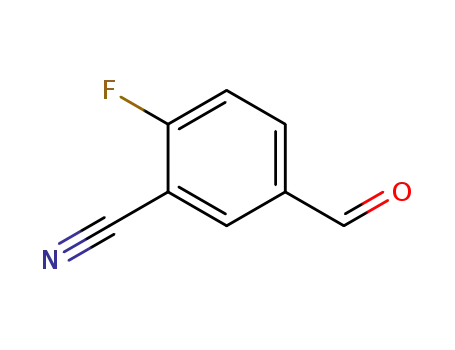
2-fluoro-5-formylbenzonitrile
763113-22-0 Downstream products
-
1613320-65-2
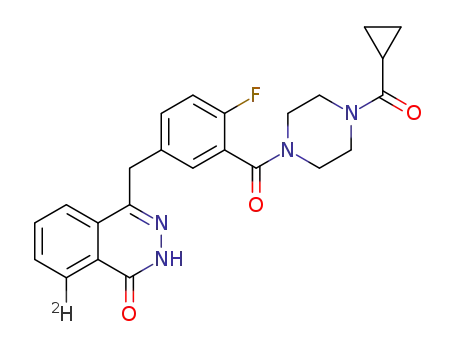
4-(3-(4-(cyclopropanecarbonyl)piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one-8-d
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Eltrombopag olamine
CAS:496775-62-3
-
Fosfomycin tromethamine
CAS:78964-85-9