1211441-98-3
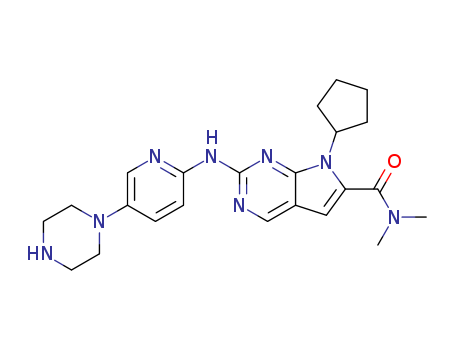
- Product Name:Ribociclib
- Molecular Formula:C23H30N8O
- Purity:99%
- Molecular Weight:434.544
Product Details:
CasNo: 1211441-98-3
Molecular Formula: C23H30N8O
Buy Quality Ribociclib,Export 1211441-98-3 Safe Delivery
- Molecular Formula:C23H30N8O
- Molecular Weight:434.544
- Vapor Pressure:0-0Pa at 20-25℃
- Boiling Point:730.8±70.0 °C(Predicted)
- PKA:8.67±0.10(Predicted)
- PSA:91.21000
- Density:1.39±0.1 g/cm3(Predicted)
- LogP:3.26320
Ribociclib(Cas 1211441-98-3) Usage
|
Description |
Fezolinetant is a neurokinin 3 (NK3) receptor antagonist, a class of medications that works by altering the nerve response that triggers the thermoregulatory center, thereby reducing vasomotor symptoms such as hot flashes. It is a small-molecule, orally active drug. |
| Uses |
Fezolinetant is used to reduce moderate to severe symptoms of menopause, including hot flashes, sweating, flushing, and chills. It is designed to reduce the frequency and severity of these symptoms by blocking neurokinin B (NKB) binding on the kisspeptin/neurokinin/dynorphin (KNDy) neuron, thus modulating neuronal activity in the hypothalamus, the brain's temperature control center. |
1211441-98-3 Relevant articles
Treatment of pre- and postmenopausal Women with HR+/HER2-metastatic Breast Cancer Ribociclib receives high AGO Recommendation Level
SM Rudesheim
, Oncology research and treatment. 2023
The use of ribociclib in the treatment of metastatic breast cancer should be determined on a case-by-case basis, taking into account factors such as disease characteristics, patient preferences, and potential side effects.
PHARMACEUTICAL COMBINATION COMPRISING TNO155 AND RIBOCICLIB
YNP Chen,H Hao,C Liu,M Mohseni
, US2022160707A1
The present invention relates to a pharmaceutical combination comprising TNO155 and ribociclib; pharmaceutical compositions comprising the same; and methods of using such combinations and compositions in the treatment or prevention of conditions in a SHP2 inhibitor combined with CDK4/6 inhibition is beneficial in, for example, the treatment of cancers.
SALT(S) OF 7-CYCLOPENTYL-2-(5-PIPERAZIN-1-YL-PYRIDIN-2-YLAMINO)-7H-PYRROLO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID DIMETHYLAMIDE AND PROCESSES OF MAKING THEREOF
-
, (2012/05/20)
This invention relates to (1) process of...
1211441-98-3 Process route
-
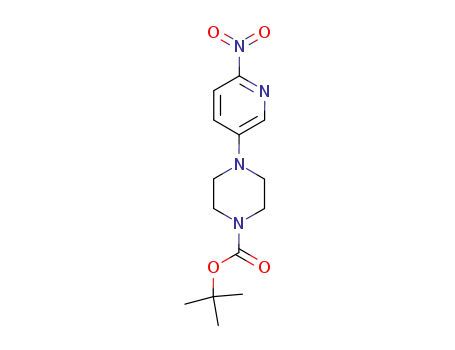
- 571189-16-7
4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester

-
![(7-cyclopentyl-Ν,Ν-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo [2,3-d]pyrimidine-6-carboxamide)](/upload/2024/1/37b2d24a-3ad5-4b30-abe5-f36dfee9c55f.png)
- 1211441-98-3
(7-cyclopentyl-Ν,Ν-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo [2,3-d]pyrimidine-6-carboxamide)
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: hydrogen / palladium 10% on activated carbon / water; methanol / 0.25 h / 19 - 54 °C / 1551.49 - 2327.23 Torr / Inert atmosphere
2.1: caesium carbonate / palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl / 4-methyl-2-pentanone / 3.25 h / 37 - 103 °C / Inert atmosphere
3.1: hydrogenchloride; water / toluene / 1.08 h / 9 - 28 °C / Inert atmosphere
3.2: Si-thiol functionalized silica gel / 6 h / 30 - 53 °C / pH 2.9 - 3.5
With hydrogenchloride; water; hydrogen; caesium carbonate; palladium 10% on activated carbon; palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In methanol; water; 4-methyl-2-pentanone; toluene;
|
|
|
Multi-step reaction with 3 steps
1.1: hydrogen / methanol / 40 °C
2.1: lithium hexamethyldisilazane / toluene / 0.5 h / Inert atmosphere
2.2: 4 h
3.1: hydrogenchloride / ethyl acetate; water / 10 h
With hydrogenchloride; hydrogen; lithium hexamethyldisilazane; In methanol; water; ethyl acetate; toluene;
|
|
|
Multi-step reaction with 3 steps
1: zinc; acetic acid / water / 2 h / 10 - 20 °C
2: sodium hydride; copper(l) iodide / acetonitrile / 1 h / 70 - 80 °C / Inert atmosphere
3: hydrogenchloride / water / 20 - 30 °C
With hydrogenchloride; copper(l) iodide; sodium hydride; acetic acid; zinc; In water; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1: zinc; acetic acid / water / 2 h / 10 - 20 °C
2: sodium hydride; copper(l) iodide / acetonitrile / 1 h / 70 - 80 °C / Inert atmosphere
3: dichloromethane / 5 - 30 °C
4: sodium hydroxide / water / pH 11 - 12
With copper(l) iodide; sodium hydride; acetic acid; sodium hydroxide; zinc; In dichloromethane; water; acetonitrile;
|
-
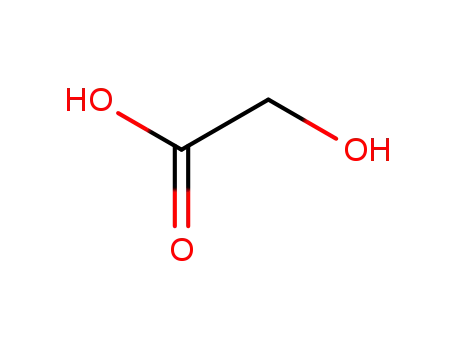
- 79-14-1
glycolic Acid

-
![(7-cyclopentyl-Ν,Ν-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo [2,3-d]pyrimidine-6-carboxamide)](/upload/2024/1/37b2d24a-3ad5-4b30-abe5-f36dfee9c55f.png)
- 1211441-98-3
(7-cyclopentyl-Ν,Ν-dimethyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrrolo [2,3-d]pyrimidine-6-carboxamide)

-
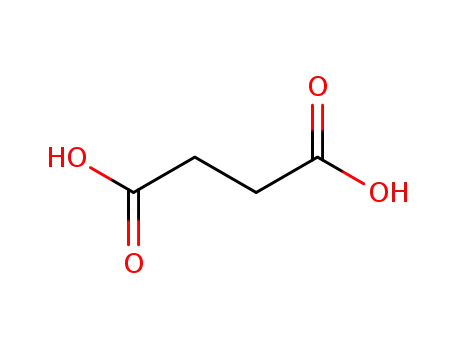
- 110-15-6
succinic acid
| Conditions | Yield |
|---|---|
|
In water; acetone; at 20 ℃;
|
1211441-98-3 Upstream products
-
571189-16-7

4-(6-nitropyridin-3-yl)-piperazine-1-carboxylic acid tert-butyl ester
1211441-98-3 Downstream products
-
1374639-75-4
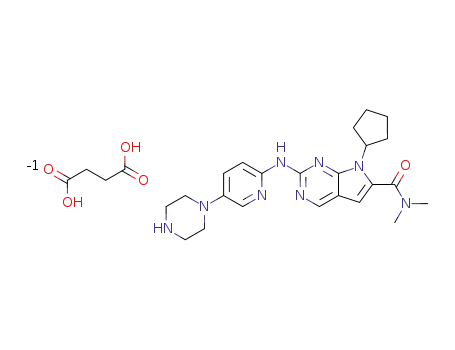
7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide succinate
-
1374639-75-4
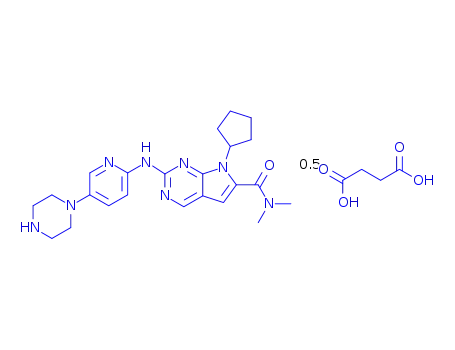
7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide hemi succinate
-
1374639-75-4
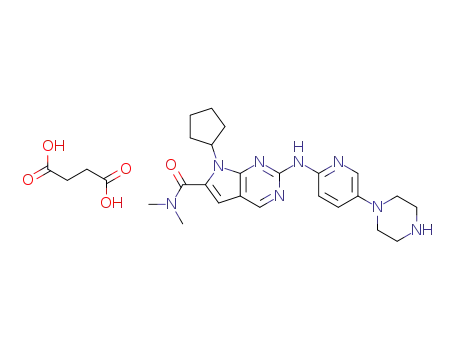
7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide succinate salt
-
110-15-6

succinic acid
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Tropisetron Hcl
CAS:105826-92-4
-
Eltrombopag olamine
CAS:496775-62-3