185243-69-0
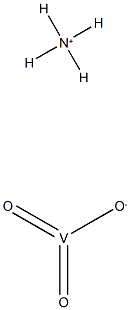
- Product Name:Etanercept
- Molecular Formula:H4NO3V
- Purity:99%
- Molecular Weight:116.97816
Product Details:
CasNo: 185243-69-0
Molecular Formula: H4NO3V
Quality Manufacturer Supply Best Quality Etanercept 185243-69-0 Safe Transportation
- Molecular Formula:H4NO3V
- Molecular Weight:116.97816
- Vapor Pressure:1.09E-05mmHg at 25°C
- Boiling Point:381.8°C at 760 mmHg
- Flash Point:178.8°C
- PSA:0.00000
- Density:1.757g/cm3
- LogP:0.00000
Etanercept(Cas 185243-69-0) Usage and Manufacturer
|
Description |
Etanercept is a medication used to manage and treat various autoimmune conditions, including plaque psoriasis, rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, and ankylosing spondylitis. Hangzhou Huarong Pharm Co., Ltd., founded in 2009, is dedicated to providing innovative products and services for small molecule drug discovery and development. The company specializes in research, development, and manufacturing across a range of fields, including Building Blocks, Reference Compounds & Impurities, Natural Products, APIs & Intermediates, and Antibody-drug Conjugates (ADCs). With a focus on R&D, Huarong Pharm has invested heavily in state-of-the-art facilities and GMP-certified plants. It has successfully served over 3,000 partners globally and aims to become a world leader in supporting life science innovation. The company’s core values are responsibility, teamwork, professionalism, efficiency, and continuous growth. |
|
Uses |
While etanercept is not classified as a traditional immunosuppressant, it does affect the immune system. By inhibiting TNF-α, etanercept can lower the ability of the immune system to fight infections. This can increase the risk of serious infections, including tuberculosis (TB), as well as infections caused by viruses, fungi, or bacteria that have spread throughout the body. Etanercept works by blocking the effects of TNF-alpha, a pro-inflammatory cytokine that becomes elevated in psoriasis, rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, and ankylosing spondylitis. |
InChI:InChI=1/C4H6N4O12/c9-5(10)17-1-3(19-7(13)14)4(20-8(15)16)2-18-6(11)12/h3-4H,1-2H2/t3-,4+
185243-69-0 Relevant articles
Etanercept A Review of its Use in Rheumatoid Arthritis
Blair Jarvis & Diana Faulds
Drugs, Volume 57, pages 945–966, (1999)
A dose-response effect was apparent with etanercept 0.25 to 16 mg/m2 twice weekly in a randomised, double-blind study in 180 patients. The mean number of swollen or tender joints at the end of the 12-week study decreased by >50% in patients treated with etanercept 16 mg/m2 twice weekly and by >25% in patients treated with placebo.
Effects of Etanercept in Patients With the Metabolic Syndrome
L. Elizabeth Bernstein, MD; Jacqueline Berry, BA; Sunnie Kim, BA;
Arch Intern Med. 2006;166(8):902-908.
Fifty-six subjects with the metabolic syndrome were randomized to administration of either etanercept or identical placebo, 50 mg subcutaneously once a week for 4 weeks. The C-reactive protein level was the primary end point. Effects on other inflammatory markers (including fibrinogen, interleukin 6, and adiponectin), insulin sensitivity, lipid levels, and body composition were also determined.
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Crisaborole(AN2728)
CAS:906673-24-3
-
Risperidone
CAS:106266-06-2