906673-24-3
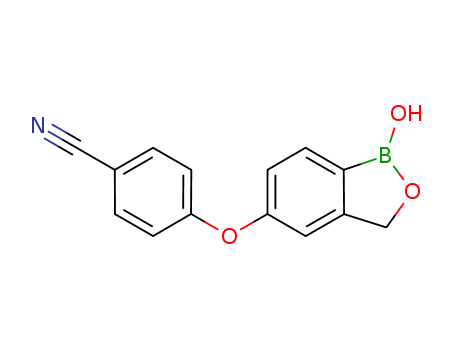
- Product Name:Crisaborole(AN2728)
- Molecular Formula:C14H10BNO3
- Purity:99%
- Molecular Weight:251.049
Product Details:
CasNo: 906673-24-3
Molecular Formula: C14H10BNO3
Buy Quality Crisaborole(AN2728),Factory Sells 906673-24-3 Efficient Shipping
- Molecular Formula:C14H10BNO3
- Molecular Weight:251.049
- Boiling Point:425.9±55.0 °C(Predicted)
- PKA:7.00±0.20(Predicted)
- PSA:62.48000
- Density:1.33±0.1 g/cm3(Predicted)
- LogP:1.56828
Crisaborole(AN2728)(Cas 906673-24-3) Usage
|
Description |
Crisaborole is a medication that treats atopic dermatitis. Crisaborole, formerly known as AN2728, is a medication belonging to the class of phosphodiesterase inhibitors. As a benzoxaborole compound, crisaborole has completed phase 3 studies and has been approved for use in the United States (December 2016) and Canada (June 2018). It is available under the brand name Eucrisa. |
| Uses | Crisaborole was approved by the U.S. Food and Drug Administration (FDA) in 2016, marking the first FDA-approved topical prescription treatment for eczema in over a decade. It is indicated for individuals with mild-to-moderate eczema and can be used on all skin tones, from nose to toes, for adults and children as young as 3 months old. This non-steroidal agent is efficacious in improving disease severity, reducing the risk of infection and reducing the signs and symptoms in patients 2 years old and older. |
| U.S. FDA Approves | Crisaborole ointment, 2%, is a novel, steroid-free, topical phosphodiesterase (PDE4) inhibitor. It is approved in the U.S. as EUCRISA® (crisaborole ointment, 2%) for topical treatment of mild-to-moderate AD in adults and pediatric patients 3 months of age and older. The safety and effectiveness of EUCRISA have been established in pediatric patients ages 3 months and older for topical treatment of mild to moderate atopic dermatitis. |
| Factory | Hangzhou Huarong Pharm Co., Ltd., founded in 2009, is dedicated to providing innovative products and services for small molecule drug discovery and development. The company specializes in research, development, and manufacturing across a range of fields, including Building Blocks, Reference Compounds & Impurities, Natural Products, APIs & Intermediates, and Antibody-drug Conjugates (ADCs). With a focus on R&D, Huarong Pharm has invested heavily in state-of-the-art facilities and GMP-certified plants. It has successfully served over 3,000 partners globally and aims to become a world leader in supporting life science innovation. The company’s core values are responsibility, teamwork, professionalism, efficiency, and continuous growth. |
906673-24-3 Relevant articles
2-Year animal carcinogenicity results for crisaborole, a novel phosphodiesterase 4 inhibitor for atopic dermatitis
Vic Ciaravino a, Dina Coronado a, Cheryl Lanphear b, Sanjay Chanda a
Journal of Dermatological Science Volume 87, Issue 2, August 2017, Pages 116-122
Crisaborole ointment, 2%, 5%, or 7%, was applied once daily topically to mice, and crisaborole was administered orally to rats at doses of 30, 100, or 300 mg/kg/day for up to 104 weeks. Systemic exposure to crisaborole and its metabolites, moribundity/death, clinical signs, and tumor formation were assessed in each study.
Crisaborole and atopic dermatitis skin biomarkers: An intrapatient randomized trial
Robert Bissonnette MD a, Ana B. Pavel PhD b, Aisleen Diaz BS b c, John L. Werth PhD d, Chuanbo Zang PhD d, Ivana Vranic MD e, Vivek S. Purohit MPharm, PhD f, Michael A. Zielinski PharmD d, Bonnie Vlahos MBA, BSN, RN d, Yeriel D. Estrada BS b, Etienne Saint-Cyr Proulx MD a, William C. Ports DVM f, Emma Guttman-Yassky MD, PhD b
Journal of Allergy and Clinical Immunology Volume 144, Issue 5, November 2019, Pages 1274-1289
Crisaborole ointment 2% is a nonsteroidal phosphodiesterase 4 inhibitor for the treatment of mild-to-moderate atopic dermatitis (AD). The mechanism of action of crisaborole and its effects on lesional measures of disease severity are not yet well defined.
906673-24-3 Upstream products
-
7732-18-5
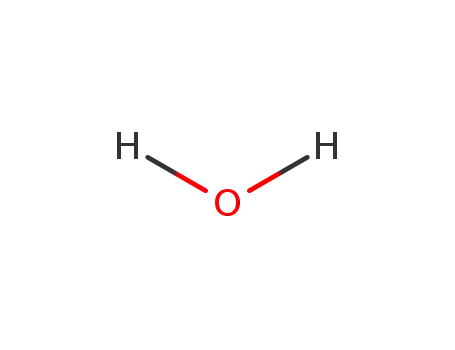
water
-
623-03-0
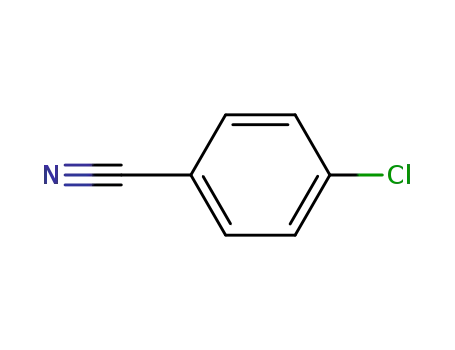
4-Cyanochlorobenzene
-
1187190-70-0
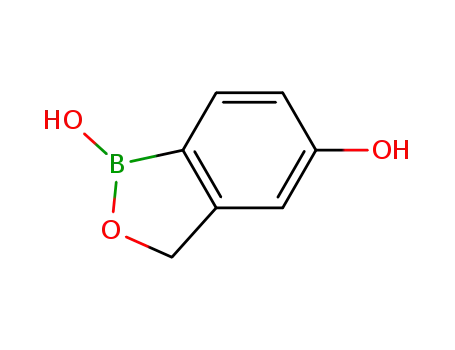
1,3-dihydro-1,5-dihydroxy-2,1-benzoxaborolane
-
623-00-7
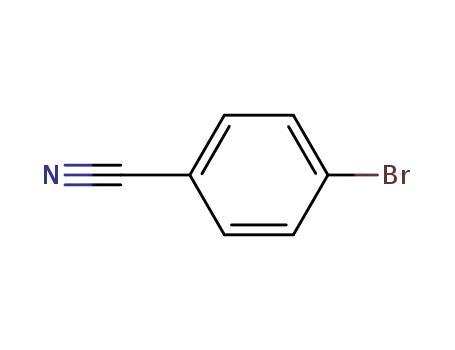
4-bromobenzenecarbonitrile
906673-24-3 Downstream products
-
947162-19-8
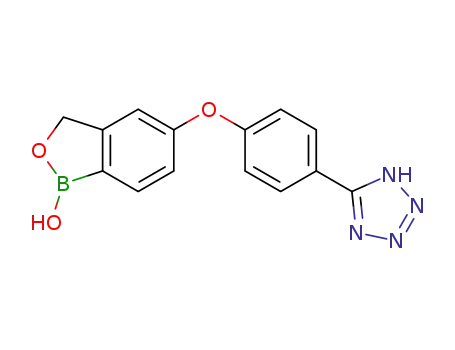
5-(4-(1H-tetrazol-5-yl)phenoxy)benzo[c][1,2]oxaborol-1(3H)-ol
-
1187187-13-8
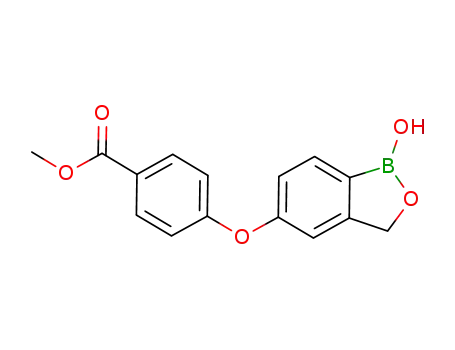
4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yloxy)benzoic acid methyl ester
Relevant Products
-
Ixazomib citrate
CAS:1201902-80-8
-
Baricitinib
CAS:1187594-09-7
-
Etanercept
CAS:185243-69-0