1187594-09-7
- Product Name:Baricitinib
- Molecular Formula:C16H17N7O2S
- Purity:99%
- Molecular Weight:371.423
Product Details:
CasNo: 1187594-09-7
Molecular Formula: C16H17N7O2S
Buy High Quality Top Purity Baricitinib 1187594-09-7 In Bulk Supply
- Molecular Formula:C16H17N7O2S
- Molecular Weight:371.423
- PKA:11.66±0.50(Predicted)
- PSA:128.94000
- Density:1.6±0.1 g/cm3
- LogP:2.11438
Baricitinib(Cas 1187594-09-7) Usage
|
Description |
Baricitinib is an oral medication belonging to the class of Janus kinase (JAK) inhibitors. It acts by inhibiting the activity of JAK proteins, particularly JAK1 and JAK2, which play a crucial role in the signaling pathways of various interleukins, interferons, and growth factors involved in inflammatory processes. |
| Uses |
Baricitinib, also known as Olumiant, is a prescription drug that treats rheumatoid arthritis by blocking the action of Janus kinase (JAK) enzymes. These enzymes are involved in the inflammation that causes rheumatoid arthritis symptoms, such as joint pain, stiffness, and swelling. Baricitinib can also slow the joint damage caused by rheumatoid arthritis. Most people who take Baricitinib notice some improvement within the first 12 weeks of treatment. Alopecia Areata: Baricitinib has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of severe alopecia areata (AA) in adults. It helps promote hair regrowth by modulating the immune response involved in AA. COVID-19: Baricitinib has been investigated for its potential use in the treatment of COVID-19. It has shown promise in reducing inflammation associated with severe COVID-19 and may also have antiviral effects by inhibiting SARS-CoV-2 endocytosis. |
| Where to buy Baricitinib | Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. |
1187594-09-7 Relevant articles
Baricitinib for the treatment of rheumatoid arthritis
Satoshi Kubo,Shingo Nakayamada &Yoshiya Tanaka
Expert Review of Clinical Immunology Volume 12, 2016 - Issue 9
Five phase 3 trials of Baricitinib, a JAK1 and JAK2 inhibitor, have been performed and showed high clinical efficacy in patients with active RA and naïve to sDMARDs or an inadequate response to sDMARDs, MTX or bDMARDs.
Baricitinib: A Review in Rheumatoid Arthritis
Zaina T. Al-Salama & Lesley J. Scott
, Drugs, Volume 78, pages 761–772, (2018)
Baricitinib was generally well tolerated during up to 5.5 years’ treatment; the most commonly reported adverse drug reactions were upper respiratory tract infections, increased LDL cholesterol, nausea and thrombocytosis.
Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact
Fabrizio Cantini Laura Niccoli Daniela Matarrese Emanuele Nicastri Paolo Stobbione Delia Goletti
, LETTERS TO THE EDITOR| VOLUME 81, ISSUE 2, P318-356, AUGUST 2020
The last consecutive patients with moderate COVID-19 pneumonia receiving standard of care therapy (lopinavir/ritonavir tablets 250 mg/bid and hydroxychloroquine 400 mg/day/orally for 2 weeks) admitted before the date of the first baricitinib-treated patient served as controls. Antibiotics were scheduled only in the case of suspected bacterial infection.
1187594-09-7 Process route
-
![tert-butyl 4-{1-[3-(cyanomethyl)-1-(ethylsulfonyl)azetidin-3-yl]-1H-pyrazol-4-yl}-7H-pyrrolo[2.3-d]pyrimidine-7-carboxylate](/upload/2024/1/12329777-a1fd-41a9-8f6d-db069dbea6c7.png)
-
tert-butyl 4-{1-[3-(cyanomethyl)-1-(ethylsulfonyl)azetidin-3-yl]-1H-pyrazol-4-yl}-7H-pyrrolo[2.3-d]pyrimidine-7-carboxylate

-
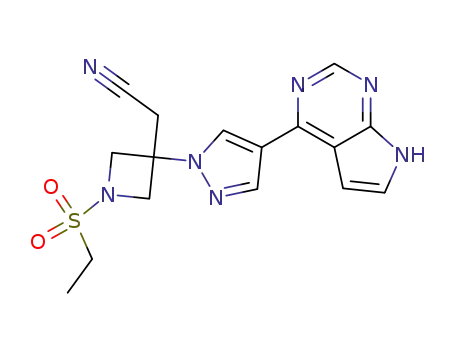
- 1187594-09-7
Baricitinib
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In ethanol; at 40 - 45 ℃;
|
93% |
|
With hydrogenchloride; In ethanol; at 40 - 45 ℃;
|
93% |
|
In water; butan-1-ol; at 90 ℃;
|
92.7% |
|
With trifluoroacetic acid; In dichloromethane; at 0 - 5 ℃; for 2h;
|
90.1% |
-
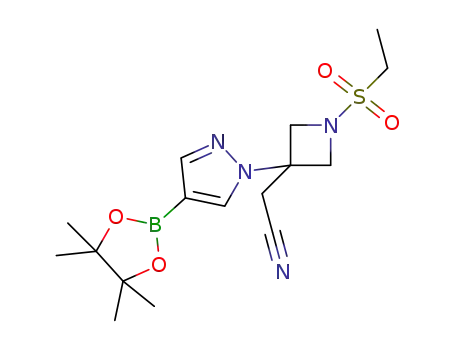
- 1919837-50-5
2-(1-(ethanesulfonyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)azetidine-3-yl)acetonitrile

-
![4-chloro-1H-pyrrolo[2,3-d]pyrimidine](/upload/2024/1/c7b935c0-1a64-49ca-9fb8-d17a135380dd.png)
- 3680-69-1
4-chloro-1H-pyrrolo[2,3-d]pyrimidine

-

- 1187594-09-7
Baricitinib
| Conditions | Yield |
|---|---|
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 80 - 85 ℃; for 5h;
|
99% |
|
With tetrakis(triphenylphosphine) palladium(0); cesium fluoride; In water; toluene; tert-butyl alcohol; at 100 ℃; for 48h; Concentration; Temperature; Solvent; Reagent/catalyst; Inert atmosphere;
|
90% |
|
With potassium phosphate; dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; In tetrahydrofuran; water; at 90 ℃; for 19h; Autoclave; Inert atmosphere;
|
90% |
|
With tetrakis(triphenylphosphine) palladium(0); cesium fluoride; In water; toluene; tert-butyl alcohol; for 48h; Reflux; Inert atmosphere;
|
84% |
1187594-09-7 Upstream products
-
1187594-13-3
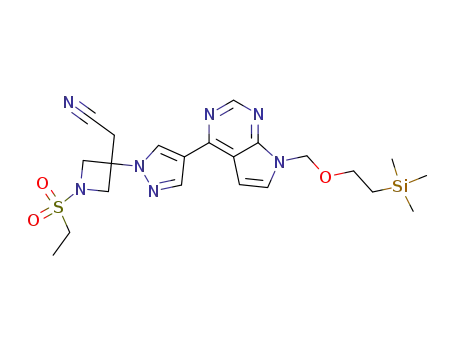
2-[1-ethanesulfonyl-3-[4-(7-[(2-(trimethylsilyl)ethoxy)methyl]-7H-pyrrolo[2,3-d]pyrimidine-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl]acetonitrile
-
1187595-90-9
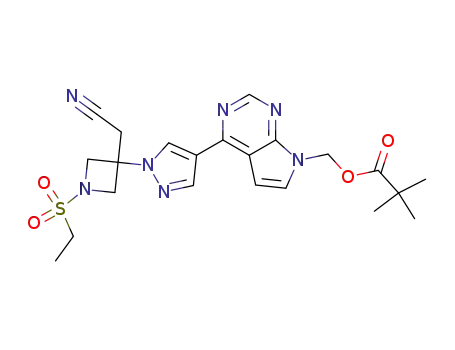
(4-{1-[3-(cyanomethyl)-1-(ethylsulphonyl)azetidin-3-yl]-1H-pyrazol-4-yl}-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl 2,2-dimethylpropanoate
-
1153949-11-1
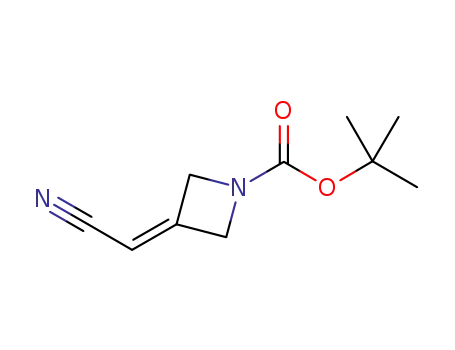
3-(cyanomethylene)azetidine-1-carboxylic acid tert-butyl ester
-
1919837-50-5

2-(1-(ethanesulfonyl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)azetidine-3-yl)acetonitrile
1187594-09-7 Downstream products
-
1187595-84-1
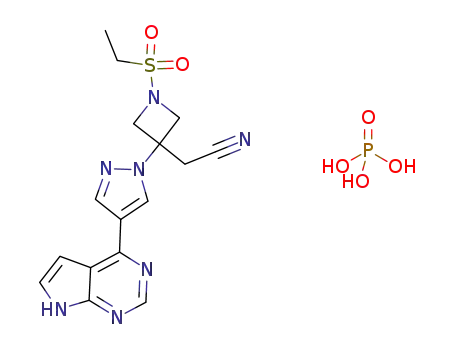
baricitinib phosphate
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Fezolinetant
CAS:1629229-37-3
-
Crisaborole(AN2728)
CAS:906673-24-3