1629229-37-3
- Product Name:Fezolinetant
- Molecular Formula:C16H15FN6OS
- Purity:99%
- Molecular Weight:0
Product Details:
CasNo: 1629229-37-3
Molecular Formula: C16H15FN6OS
Buy Quality Fezolinetant,Factory Sells 1629229-37-3 Safe Shipping
- Molecular Formula:
- Molecular Weight:0
Fezolinetant(Cas 1629229-37-3) Usage
|
Description |
Fezolinetant is a neurokinin 3 (NK3) receptor antagonist, a class of medications that works by altering the nerve response that triggers the thermoregulatory center, thereby reducing vasomotor symptoms such as hot flashes. It is a small-molecule, orally active drug. |
| Uses |
Fezolinetant is used to reduce moderate to severe symptoms of menopause, including hot flashes, sweating, flushing, and chills. It is designed to reduce the frequency and severity of these symptoms by blocking neurokinin B (NKB) binding on the kisspeptin/neurokinin/dynorphin (KNDy) neuron, thus modulating neuronal activity in the hypothalamus, the brain's temperature control center. |
1629229-37-3 Relevant articles
Fezolinetant for menopausal hot flashes and night sweats
I Gonzalez-Garcia,M Lopez
, Trends in pharmacological sciences, 2023
Clinical trials have shown promising results for fezolinetant in the treatment of menopausal symptoms, with some studies demonstrating significant reductions in the frequency and severity of hot flashes and night sweats compared to placebo.
Fezolinetant. Tachykinin NK3 receptor antagonist, Treatment of menopausal-related vasomotor symptoms
G D'Alessando,F Barra,G Evangelisti,S Ferrero
, Drugs of the Future 2020/01/01
It selectively and reversibly blocks neurokinin B (NKB) signaling, leading to a relevant beneficial effect for the treatment of flushes. A phase II double-blind, placebocontrolled study showed that fezolinetant significantly decreases the severity of VMS, inducing a 93% reduction in their frequency from baseline to week 12 of treatment.
1629229-37-3 Process route
-
![(R)-3-methyl-5-(8-methyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)-1,2,4-thiadiazole hydrochloride](/upload/2024/1/d56b764c-1a1e-4590-bd3a-898f522e3857.png)
-
(R)-3-methyl-5-(8-methyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)-1,2,4-thiadiazole hydrochloride

-
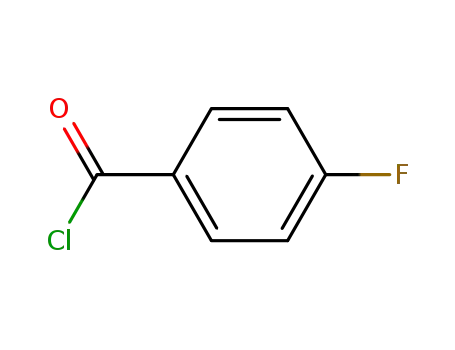
- 403-43-0
4-fluorobenzoyl chloride

-
![(R)-(4-fluorophenyl)-(8-methyl-3-(3-(methyl)-1,2,4-thiadiazol-5-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone](/upload/2024/1/6de3cb31-4216-42a6-afd1-fa79953383a5.png)
- 1629229-37-3
(R)-(4-fluorophenyl)-(8-methyl-3-(3-(methyl)-1,2,4-thiadiazol-5-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone
| Conditions | Yield |
|---|---|
|
With sodium hydrogencarbonate; In dichloromethane; at 20 ℃; for 1h; Inert atmosphere;
|
65% |
-
![(R)-5-(7-(2,4-dimethoxybenzyl)-8-methyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)-3-methyl-1,2,4-thiadiazole](/upload/2024/1/961cbb85-7898-41d9-9095-0e8a8da29061.png)
-
(R)-5-(7-(2,4-dimethoxybenzyl)-8-methyl-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)-3-methyl-1,2,4-thiadiazole

-
![(R)-(4-fluorophenyl)-(8-methyl-3-(3-(methyl)-1,2,4-thiadiazol-5-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone](/upload/2024/1/6de3cb31-4216-42a6-afd1-fa79953383a5.png)
- 1629229-37-3
(R)-(4-fluorophenyl)-(8-methyl-3-(3-(methyl)-1,2,4-thiadiazol-5-yl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: trifluoroacetic acid / dichloromethane / 0.42 h / 0 - 20 °C / Inert atmosphere
2: sodium hydrogencarbonate / dichloromethane / 1 h / 20 °C / Inert atmosphere
With sodium hydrogencarbonate; trifluoroacetic acid; In dichloromethane;
|
1629229-37-3 Upstream products
-
1383146-20-0
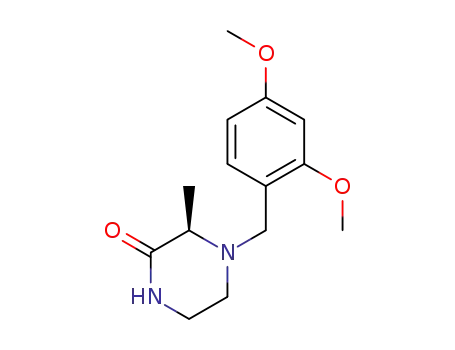
(R)-4-(2,4-dimethoxybenzyl)-3-methylpiperazin-2-one
-
403-43-0

4-fluorobenzoyl chloride
-
1429560-52-0
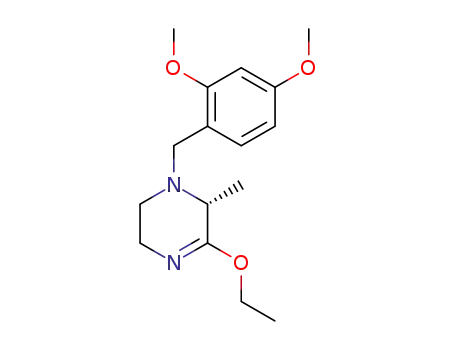
(R)-1-(2,4-dimethoxybenzyl)-5-ethoxy-6-methyl-1,2,3,6-tetrahydropyrazine
Relevant Products
-
Apalutamide
CAS:956104-40-8
-
Ciclopirox
CAS:29342-05-0
-
Baricitinib
CAS:1187594-09-7