956104-40-8
- Product Name:Apalutamide
- Molecular Formula:C21H15F4N5O2S
- Purity:99%
- Molecular Weight:477.442
Product Details:
CasNo: 956104-40-8
Molecular Formula: C21H15F4N5O2S
Quality Manufacturer Supply Apalutamide,Offer 956104-40-8 Fast Delivery
- Molecular Formula:C21H15F4N5O2S
- Molecular Weight:477.442
- PKA:13.83±0.46(Predicted)
- PSA:121.42000
- Density:1.59±0.1 g/cm3(Predicted)
- LogP:4.05248
Apalutamide(Cas 956104-40-8) Usage
|
Description |
Apalutamide, marketed under the brand name ErleadaTM, is a next-generation oral androgen receptor (AR) inhibitor developed by Janssen for the treatment of prostate cancer (PC). It belongs to the class of medications called androgen receptor inhibitors. |
| Uses |
Apalutamide received its first global approval in the USA in February 2018 for the treatment of nmCRPC. This stage of prostate cancer is characterized by rising prostate-specific antigen (PSA) levels despite castrate levels of testosterone and no evidence of metastatic disease. It works by blocking the effects of androgen (a male reproductive hormone) to stop the growth and spread of cancer cells. Giving golimumab and apalutamide may work better in treating patients with castration-resistant prostate cancer. Apalutamide is typically continued until disease progression, unacceptable toxicity, or patient choice to stop treatment. Decisions regarding the continuation of treatment should be made in consultation with a healthcare provider and may occur at least by the start of the third 4-weekly cycle of treatment. |
| Mechanism of action | Apalutamide is an androgen receptor (AR) antagonist that binds to ARs in target tissues, preventing them from being activated by androgens. This prevents the binding of ARs to AR-responsive genes, which inhibits the expression of genes that regulate prostate cancer cell proliferation. |
| Quality Manufacturer | Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. At present, Huarong Pharm has successfully delivered innovative R&D products and services to more than 3,000 partners across the world. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
956104-40-8 Relevant articles
Apalutamide: the established and emerging roles in the treatment of advanced prostate cancer
Athanasios E. Dellis &Athanasios G. Papatsoris
, Expert Opinion on Investigational Drugs Volume 27, 2018 - Issue 6
The authors review Phase I, II, and III studies for apalutamide, in a large spectrum of PCa (from low-risk to metastatic CRPC [mCRPC]) patients as sole treatment or in the setting of combined therapy.
Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone
Dana E. Rathkopf; Emmanuel S. Antonarakis; Neal D. Shore; Ronald F. Tutrone; Joshi J. Alumkal; Charles J. Ryan; Mansoor Saleh; Ralph J. Hauke; Rajesh Bandekar; Edna Chow Maneval; Carla J. de Boer; Margaret K. Yu; Howard I. Scher
, Clinical Cancer Research, Volume 23, Issue 14 15 July 2017
All received apalutamide 240 mg/day. Primary endpoint was ≥50% decline in 12-week PSA according to Prostate Cancer Working Group 2 criteria. Secondary endpoints included time to PSA progression and time on treatment.
956104-40-8 Process route
-
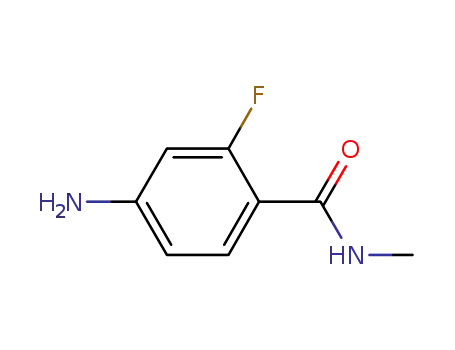
- 915087-25-1
2-fluoro-N-methyl-4-amino-benzamide

-
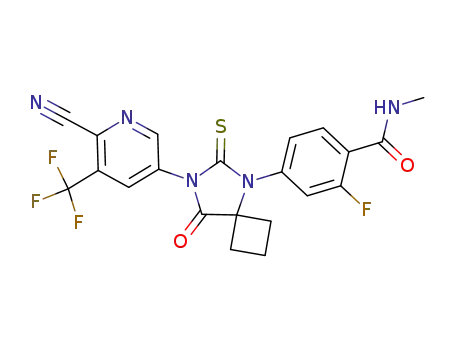
- 956104-40-8
ARN-509
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1.1: acetic acid / 16 h / 80 °C
2.1: N,N-dimethyl acetamide / 16 h / 60 °C
2.2: 1 h / Reflux
With acetic acid; In N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 2 steps
1.1: acetic acid / 24 h / 80 °C
2.1: N,N-dimethyl-formamide / 20 h / 80 °C / Microwave irradiation
2.2: 2 h
With acetic acid; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1.1: acetic acid / 16 h / 80 °C / Sealed tube
2.1: N,N-dimethyl acetamide / 16 h / 60 °C / Inert atmosphere
2.2: 2 h / Reflux; Inert atmosphere
With acetic acid; In N,N-dimethyl acetamide;
|
|
|
Multi-step reaction with 2 steps
1.1: triethylamine / dichloromethane / 20 h / 20 °C
1.2: 4 h / Reflux
1.3: 16 h / 20 °C
2.1: N,N-dimethyl-formamide / 16 h / 80 °C / Sealed tube
With triethylamine; In dichloromethane; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: triethylamine / dichloromethane / 20 h / 20 °C
1.2: 4 h / Reflux
1.3: 16 h / 20 °C
2.1: thionyl chloride / 16 h / 0 - 40 °C
3.1: pyridine / 4.75 h / 60 °C
With pyridine; thionyl chloride; triethylamine; In dichloromethane;
|
|
|
Multi-step reaction with 3 steps
1.1: triethylamine / dichloromethane / 20 h / 20 °C
1.2: 4 h / Reflux
1.3: 16 h / 20 °C
2.1: dicyclohexyl-carbodiimide / dichloromethane; ethyl acetate / 2 h / -15 - 0 °C
3.1: pyridine / 112 h / 20 - 60 °C / Sealed tube
With pyridine; triethylamine; dicyclohexyl-carbodiimide; In dichloromethane; ethyl acetate;
|
|
|
Multi-step reaction with 2 steps
1.1: acetic acid / tetrahydrofuran / 26.17 h / 25 - 85 °C
2.1: Isopropyl acetate; N,N-dimethyl-formamide / 65 - 70 °C
2.2: 3 h / 25 - 85 °C
With acetic acid; In tetrahydrofuran; Isopropyl acetate; N,N-dimethyl-formamide;
|
-
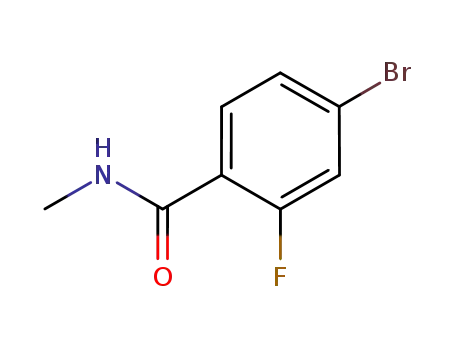
- 749927-69-3
4-bromo-2-fluoro-N-methylbenzanamide

-

- 956104-40-8
ARN-509
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2.1: potassium carbonate / N,N-dimethyl-formamide / 0.17 h / 50 °C
2.2: 10 h / 0 - 20 °C
3.1: dimethyl sulfoxide / 1 h / 85 °C / Inert atmosphere
With potassium carbonate; In water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2.1: potassium carbonate / N,N-dimethyl-formamide / 0.17 h / 50 °C
2.2: 5 h / 50 °C
3.1: dimethyl sulfoxide / 85 °C / Inert atmosphere
With potassium carbonate; In water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2.1: dmap / dichloromethane / 0.17 h
2.2: 24 h / 0 - 20 °C
3.1: dimethyl sulfoxide / 8 h / 85 °C / Inert atmosphere
With dmap; potassium carbonate; In dichloromethane; water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2.1: dmap / dichloromethane / 0.17 h
2.2: 24 h / 0 - 20 °C
3.1: dimethyl sulfoxide / 85 °C / Inert atmosphere
With dmap; potassium carbonate; In dichloromethane; water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2: potassium carbonate / N,N-dimethyl-formamide; water / 1 h / 30 - 40 °C
3: dimethyl sulfoxide / 8 h / 80 °C
With potassium carbonate; In water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide; water / 18 h / 100 °C
2: potassium carbonate / N,N-dimethyl-formamide; water / 5 h / 30 - 50 °C
3: dimethyl sulfoxide / 5 h / 85 °C
With potassium carbonate; In water; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: 2-acetylcyclohexanone; potassium carbonate; copper(l) chloride / N,N-dimethyl acetamide; water / 8 h / 95 - 105 °C / Inert atmosphere
2: potassium carbonate / N,N-dimethyl acetamide / 1 h / 20 - 45 °C / Inert atmosphere
3: acetonitrile / 40 h / 85 °C
With 2-acetylcyclohexanone; potassium carbonate; copper(l) chloride; In N,N-dimethyl acetamide; water; acetonitrile;
|
|
|
Multi-step reaction with 4 steps
1: caesium carbonate; copper(l) iodide / N,N-dimethyl acetamide / 90 - 95 °C / Inert atmosphere
2: thionyl chloride / 40 - 45 °C
3: N-ethyl-N,N-diisopropylamine / methanol / Reflux
4: potassium carbonate; copper(l) iodide / N,N-dimethyl-formamide / 100 - 110 °C / Inert atmosphere
With copper(l) iodide; thionyl chloride; potassium carbonate; caesium carbonate; N-ethyl-N,N-diisopropylamine; In methanol; N,N-dimethyl acetamide; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 2 steps
1: copper(l) iodide; potassium carbonate; triethylamine; 2-acetylcyclohexanone / water; N,N-dimethyl-formamide / 60 h / 95 - 100 °C
2: N,N-dimethyl-formamide / 16 h / 80 °C / Sealed tube
With copper(l) iodide; 2-acetylcyclohexanone; potassium carbonate; triethylamine; In water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: copper(l) iodide; potassium carbonate; triethylamine; 2-acetylcyclohexanone / water; N,N-dimethyl-formamide / 60 h / 95 - 100 °C
2: thionyl chloride / 16 h / 0 - 40 °C
3: pyridine / 4.75 h / 60 °C
With pyridine; copper(l) iodide; thionyl chloride; 2-acetylcyclohexanone; potassium carbonate; triethylamine; In water; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: copper(l) iodide; potassium carbonate; triethylamine; 2-acetylcyclohexanone / water; N,N-dimethyl-formamide / 60 h / 95 - 100 °C
2: dicyclohexyl-carbodiimide / dichloromethane; ethyl acetate / 2 h / -15 - 0 °C
3: pyridine / 112 h / 20 - 60 °C / Sealed tube
With pyridine; copper(l) iodide; 2-acetylcyclohexanone; potassium carbonate; triethylamine; dicyclohexyl-carbodiimide; In dichloromethane; water; ethyl acetate; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1.1: potassium carbonate; copper(l) chloride / N,N-dimethyl-formamide / 10.5 h / 20 - 110 °C / Inert atmosphere
2.1: potassium carbonate / N,N-dimethyl-formamide / 0.5 h / 20 °C
2.2: 2 h / 40 °C
3.1: N,N-dimethyl-formamide / 8 h / 85 °C / Inert atmosphere
With potassium carbonate; copper(l) chloride; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; copper(l) chloride / N,N-dimethyl-formamide / 10.5 h / 20 - 110 °C / Inert atmosphere
2: sulfuric acid / 20 h / Reflux
3: N,N-dimethyl-formamide / 8 h / 85 °C / Inert atmosphere
With sulfuric acid; potassium carbonate; copper(l) chloride; In N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; copper(l) chloride / N,N-dimethyl-formamide / 10.5 h / 20 - 110 °C / Inert atmosphere
2: acetyl chloride / 10 h / Reflux
3: dimethylsulfoxide-d6 / 12 h / 85 °C / Inert atmosphere
With potassium carbonate; acetyl chloride; copper(l) chloride; In dimethylsulfoxide-d6; N,N-dimethyl-formamide;
|
|
|
Multi-step reaction with 3 steps
1: potassium carbonate; copper(l) chloride / N,N-dimethyl-formamide / 10.5 h / 20 - 110 °C / Inert atmosphere
2: acetyl chloride / 10 h / Reflux
3: dimethyl sulfoxide; Isopropyl acetate / 12 h / 85 °C / Inert atmosphere
With potassium carbonate; acetyl chloride; copper(l) chloride; In Isopropyl acetate; dimethyl sulfoxide; N,N-dimethyl-formamide;
|
956104-40-8 Upstream products
-
573762-62-6
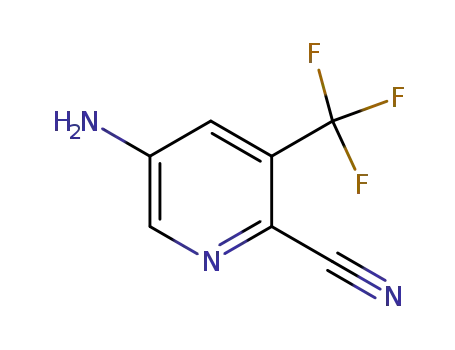
5-amino-3-(trifluoromethyl)pyridine-2-carbonitrile
-
915087-26-2
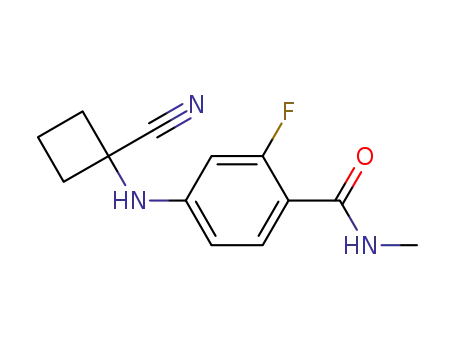
4-(1-cyano-cyclobutanylamino)-2-fluoro-N-methyl-benzamide
-
463-71-8
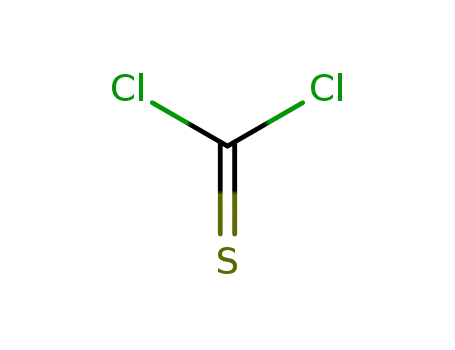
thiophosgene
-
573762-57-9
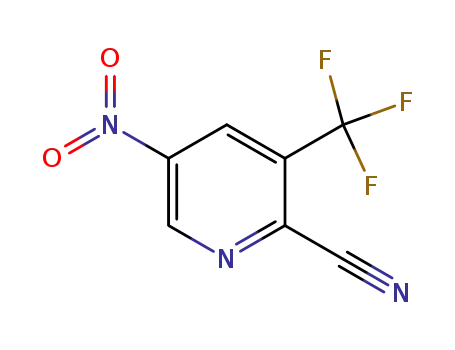
2-cyano-3-(trifluoromethyl)-5-nitropyridine
Relevant Products
-
Fezolinetant
CAS:1629229-37-3
-
Upadacitinib
CAS:1310726-60-3
-
Adagrasib
CAS:2326521-71-3