1310726-60-3
- Product Name:Upadacitinib
- Molecular Formula:C17H19F3N6O
- Purity:99%
- Molecular Weight:380.373
Product Details:
CasNo: 1310726-60-3
Molecular Formula: C17H19F3N6O
Buy Reliable Quality Upadacitinib 1310726-60-3 On Stock
- Molecular Formula:C17H19F3N6O
- Molecular Weight:380.373
- PKA:11.89±0.60(Predicted)
- PSA:78.32000
- Density:1.56±0.1 g/cm3(Predicted)
- LogP:3.23670
Upadacitinib 1310726-60-3 Usage
|
Description |
Upadacitinib, sold under the brand name Rinvoq, is an immunosuppressant medication approved by the FDA for treating various conditions. As a second-generation Janus kinase (JAK) inhibitor specifically targeting the JAK1 enzyme, Upadacitinib works by reducing the activity of the immune system. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. At present, Huarong Pharm has successfully delivered innovative R&D products and services to more than 3,000 partners across the world. Our goal is to become a world-class leading company to support life science innovation and manufacturing. |
|
Uses |
It is used to treat the following conditions: Moderate to severe rheumatoid arthritis |
1310726-60-3 Relevant articles
Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial
Prof Gerd R Burmester, MD Prof Joel M Kremer, MD Prof Filip Van den Bosch, MD Alan Kivitz, MD Prof Louis Bessette, MD Yihan Li, PhD
The Lancet, VOLUME 391, ISSUE 10139, P2503-2512, JUNE 23, 2018
Using interactive response technology, we randomly assigned patients receiving stable background csDMARDs (2:2:1:1) to receive a once-daily extended-release formulation of upadacitinib 15 mg or 30 mg, or placebo, for 12 weeks. Patients, investigators, and the funder were masked to allocation.
Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study
Prof Josef S Smolen, FRCPa josef.smolen@meduniwien.ac.at ∙ Aileen L Pangan, MDb ∙ Prof Paul Emery, FMedScic ∙ Prof William Rigby, MDd ∙ Prof Yoshiya Tanaka, MDe ∙ Juan Ignacio Vargas, MDf∙
, The Lancet, June 27, 2019
Adverse events were reported in 102 patients (47%) on continued methotrexate, 103 (47%) on upadacitinib 15 mg, and 105 (49%) on upadacitinib 30 mg. Herpes zoster was reported by one (<1%) patient on continued methotrexate, three (1%) on upadacitinib 15 mg, and six (3%) on upadacitinib 30 mg. Three malignancies (one [<1%] on continued methotrexate, two [1%] on upadacitinib 15 mg), three adjudicated major adverse cardiovascular events (one [<1%] on upadacitinib 15 mg, two [<1%] on upadacitinib 30 mg), one adjudicated pulmonary embolism (<1%; upadacitinib 15 mg), and one death (<1%; upadacitinib 15 mg, haemorrhagic stroke [ruptured aneurysm]) were reported in the study.
PYRROLIDINE COMPOUNDS, ITS SALT AND USE IN THE PREPARATION OF UPADACITINIB THEREOF
-
, (2021/01/29)
The present invention relates to process...
1310726-60-3 Upstream products
-
753-90-2
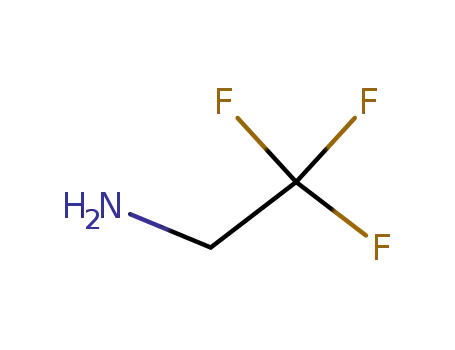
trifluoroethylamine
-
530-62-1
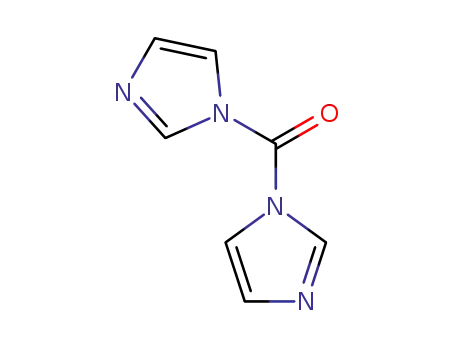
1,1'-carbonyldiimidazole
-
1428243-26-8
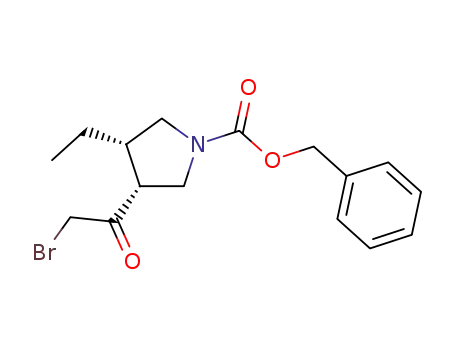
(3R,4S)-benzyl 3-(2-bromoacetyl)-4-ethylpyrrolidine-1-carboxylate
-
1201186-54-0
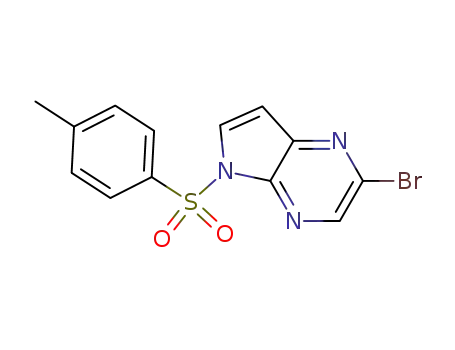
2-bromo-5-(p-toluenesulfonyl)-5H-pyrrolo[2,3-b]pyrazine
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Resmetirom
CAS:920509-32-6
-
Apalutamide
CAS:956104-40-8