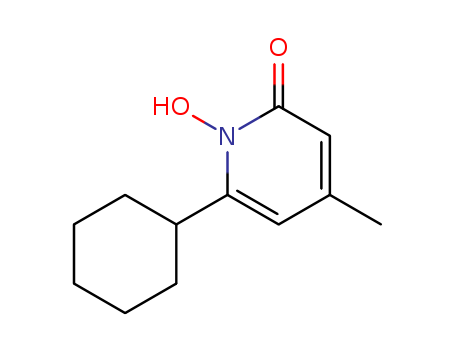
29342-05-0
- Product Name:Ciclopirox
- Molecular Formula:C12H17NO2
- Purity:99%
- Molecular Weight:207.272
Product Details:
CasNo: 29342-05-0
Molecular Formula: C12H17NO2
Appearance: white or light-yellow powder
Buy High Grade Ciclopirox 29342-05-0,Wholesale 29342-05-0 Safe Transportation
- Molecular Formula:C12H17NO2
- Molecular Weight:207.272
- Appearance/Colour:white or light-yellow powder
- Vapor Pressure:2.71E-06mmHg at 25°C
- Melting Point:144 °C
- Refractive Index:1.582
- Boiling Point:350 °C at 760 mmHg
- PKA:6.25±0.58(Predicted)
- Flash Point:165.5 °C
- PSA:42.23000
- Density:1.193 g/cm3
- LogP:2.44170
Ciclopirox(Cas 29342-05-0) Usage
|
Description |
Ciclopirox (CPX) is classified as an antifungal agent and is primarily used as a topical antimycotic agent for treating fungal infections of the skin and nails. It is also being explored as a potential anticancer agent due to its emerging effects on tumor growth inhibition. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm has built platforms for the research, development and manufacturing of Building Blocks, Reference Compounds & Impurities, Natural products, APIs & Intermediates, Antibody-drug Conjugates (ADCs) and others. To maintain a high innovation efficiency, the company has continuously increased the investment on R&D facilities and state-of-the-art equipment in the past several years, including the establishment of kilogram GMP conditions plants and R&D centers. |
|
Uses |
Ciclopirox is a synthetic hydroxypyridone compound used to treat fungal infections, particularly those affecting the nails and skin. Unlike common antifungal agents like azoles, Ciclopirox works by inhibiting the growth of fungi and yeasts by blocking essential enzymatic functions and cellular processes. It also has emerging roles as an anti-inflammatory and anticancer agent. Research has shown that Ciclopirox exhibits activity against various cancers, including colorectal cancer (CRC), rhabdomyosarcoma, leukemia, and neuroblastoma. It works by inhibiting key cellular pathways involved in tumor growth, inducing apoptosis through ROS-mediated ER stress, and disrupting iron metabolism in cancer cells. |
|
Originator |
Fungirox Esmalte, UCI-Farma |
| Advantages | Broad-spectrum antifungal activity: Effective against a wide variety of fungi, including those resistant to common antifungal treatments. Emerging anticancer applications: Shows potential in inhibiting cancer cell growth and inducing cell death, particularly in resistant cancer cell types. Anti-inflammatory properties: Useful in reducing inflammation associated with fungal infections. |
InChI:InChI=1/C12H17NO2/c1-9-7-11(13(15)12(14)8-9)10-5-3-2-4-6-10/h7-8,10,15H,2-6H2,1H3
29342-05-0 Relevant articles
Ciclopirox Recent Nonclinical and Clinical Data Relevant to its Use as a Topical Antimycotic Agent
Alessandro Subissi, Daniela Monti, Giuseppe Togni & Federico Mailland
Drugs, Volume 70, pages 2133–2152, (2010)
As a general conclusion, although less effective than some oral antimycotic agents in various indications, ciclopirox compares very well in terms of the benefit/risk ratio due to its excellent tolerability and complete absence of serious adverse effects.
The antitumor activity of the fungicide ciclopirox
Hongyu Zhou, Tao Shen, Yan Luo, Lei Liu, Wenxing Chen, Baoshan Xu, Xiuzhen Han, Jia Pang, Chantal A. Rivera, Shile Huang
International Journal of Cancer, Volume127, Issue10 15 November 2010 Pages 2467-2477
To assess the antitumor effect of Ciclopirox(CPX), human breast cancer MDA-MB231 xenografts were established in BALB/c nu/nu mice. When tumors reached <200 mm3, mice were divided randomly into CPX treatment group and control group (5 mice/group). Subsequently, mice were treated daily by oral gavages with CPX (25 mg/kg) prepared in a solution (4% ethanol, 5.2% Tween 80 and 5.2% PEG 400) or vehicle control.
A DFT study of Se-decorated B12N12 nanocluster as a possible drug delivery system for ciclopirox
Sadegh Kaviani a , Siyamak Shahab b c d , Masoome Sheikhi e , Vladimir Potkin d , Hongwei Zhou f
Computational and Theoretical Chemistry Volume 1201 , July 2021, 113246
Hence, the enhancement of ciclopirox polarity is crucial in biological systems. In this work, the effect of selenium decoration on the interaction of ciclopirox drug molecule with B12N12 was investigated using density functional theory (DFT) calculations. Finally, electronic properties analysis showed that energy band gap of Se-B12N12 nanocluster is significantly reduced from 0.2835 to 0.2121 a.u after ciclopirox adsorption.
29342-05-0 Upstream products
-
14818-35-0
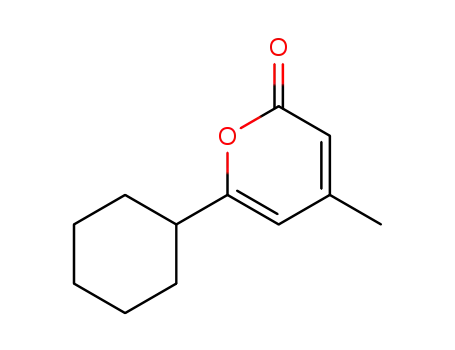
4-methyl-6-cyclohexyl02-pyrone
-
924-50-5
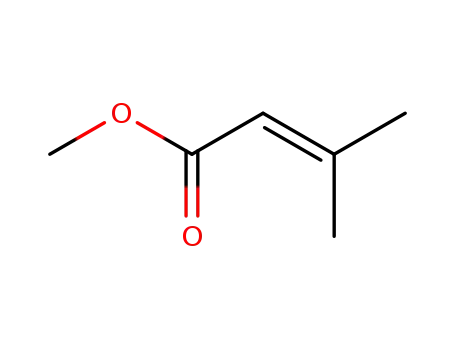
Methyl 3,3-dimethylacrylate
-
2719-27-9
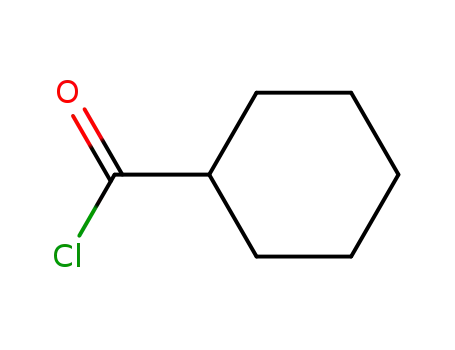
cyclohexanylcarbonyl chloride
-
288-32-4
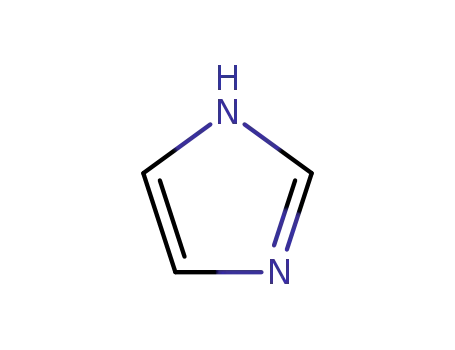
1H-imidazole
29342-05-0 Downstream products
-
1351572-55-8
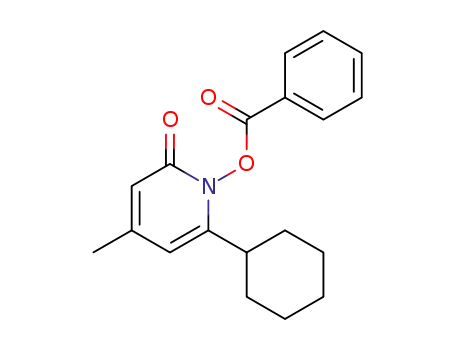
1-benzoyloxy 6-cyclohexyl-4-methylpyridine-2(1H)-one
-
41621-49-2
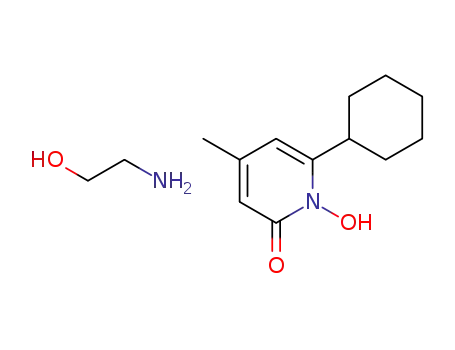
cyclopirox olamine
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Brexpiprazole
CAS:913611-97-9
-
Fezolinetant
CAS:1629229-37-3