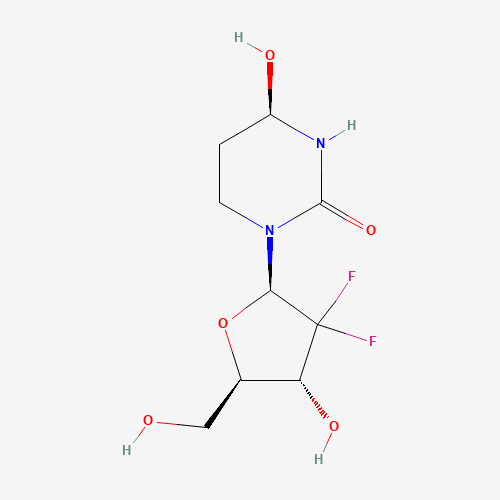
1141397-80-9
- Product Name:Cedazuridine
- Molecular Formula:C9H14F2N2O5
- Purity:99%
- Molecular Weight:268.21
Product Details:
CasNo: 1141397-80-9
Molecular Formula: C9H14F2N2O5
Buy Quality Cedazuridine,Reliable Quality 1141397-80-9 Fast Shipping
- Molecular Formula:C9H14F2N2O5
- Molecular Weight:268.21
- Melting Point:162-165
Cedazuridine(Cas 1141397-80-9) Usage
|
Description |
Cedazuridine is a cytidine deaminase inhibitor, which means it prevents the breakdown of decitabine, a DNA methylase inhibitor used in the treatment of MDS and CMML. Cedazuridine is sold under the brand name Inqovi, usually as a fixed-dose combination with decitabine. |
| Uses |
Cedazuridine, in combination with decitabine, is indicated for the treatment of: Myelodysplastic syndromes (MDS): A group of cancers characterized by ineffective production of blood cells in the bone marrow. |
1141397-80-9 Relevant articles
LOW DOSE COMBINATION CDA SUBSTRATE DRUG/CEDAZURIDINE WITH EXTENDED ADMINISTRATION
H Keer,M Azab,A Oganesian
, -
This invention relates to methods and compositions for administering an effective amount of a CDA substrate drug and an effective amount of cedazuridine. In particular, the invention relates to methods for treating cancer, inhibiting degradation of a CDA substrate drug, and reducing DNA methylation in a subject in need thereof comprising administering an effective amount of a CDA substrate drug and an effective amount of cedazuridine.
Clinical Efficacy and Safety of Oral Decitabine/Cedazuridine in 133 Patients with Myelodysplastic Syndromes (MDS) and Chronic Myelomonocytic Leukemia (CMML)
M Savona,JK Mccloskey,E Griffiths,GG Manero
Blood, 2020
Hypomethylating agents (HMAs) or DNA methyltransferase inhibitors (DNMTi) such as decitabine or azacitidine are established standard of care for the treatment of MDS and CMML. The oral bioavailability of these agents has been limited due to rapid degradation by cytidine deaminase (CDA) in the gut and liver, hence requiring intravenous infusion or subcutaneous injections daily for 5-7 days every month (m).
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Mirogabalin besylate
CAS:1138245-21-2
-
Abiraterone Acetate
CAS:154229-18-2