1138245-21-2
- Product Name:Mirogabalin besylate
- Molecular Formula:C18H25NO5S
- Purity:99%
- Molecular Weight:367.466
Product Details:
CasNo: 1138245-21-2
Molecular Formula: C18H25NO5S
Buy High Quality Mirogabalin besylate 1138245-21-2 Competitive Price
- Molecular Formula:C6H6O3S*C12H19NO2
- Molecular Weight:367.466
Mirogabalin besylate(Cas 1138245-21-2) Usage
|
Description |
Mirogabalin besylate belongs to the class of drugs known as gabapentinoids. It is formulated as film-coated tablets for oral administration. |
| Uses |
Mirogabalin besylate is approved in Japan for the treatment of two types of neuropathic pain: Postherpetic neuralgia: This condition refers to nerve pain that persists after a shingles (herpes zoster) outbreak has healed. |
1138245-21-2 Relevant articles
Mirogabalin besylate in the treatment of neuropathic pain
Burgess, J.Javed, S.Frank, B.Malik, R. A.Alam, U.
Drugs of today: Medicamentos de actualidad, 2020
Mirogabalin has a potent pain-modulating effect with a unique high affinity and prolonged dissociation rate for the alpha(2)delta-1 subunit of voltage-gated calcium (Ca2+) channels (VGCCs) on the dorsal root ganglion resulting in more sustained analgesia compared with traditional gabapentinoids.
Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases
K Karatsu,R Tamura,T Miyauchi,J Sogano,U Hino,T Iwama,M Toda
Page/Page column 52, (2010/06/17)
Mirogabalin besylate is a selective ligand for the α2δ subunit of voltage-gated calcium channels. Although mirogabalin has been used for patients with postherpetic neuralgia and painful diabetic peripheral neuropathy, few reports have assessed the effect on postsurgical neuropathy.
1138245-21-2 Process route
-
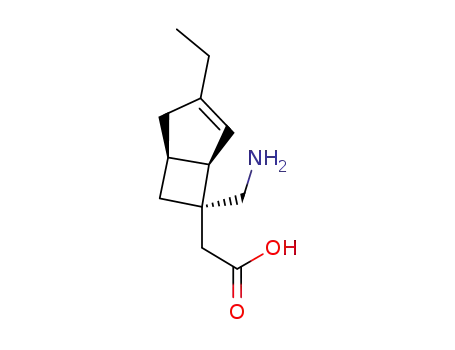
- 1138245-13-2
DS-5565

-
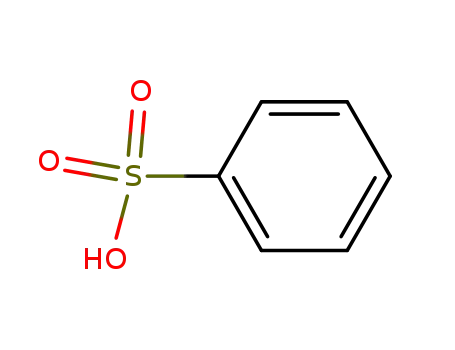
- 98-11-3
benzenesulfonic acid

-
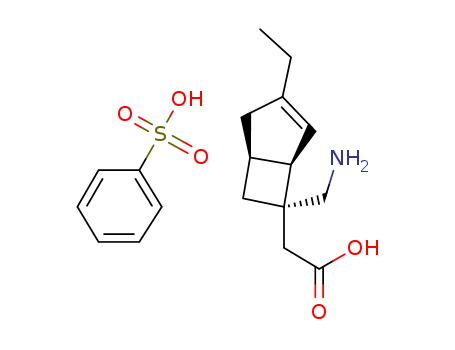
![[(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate](/upload/2024/1/d20df947-c9cc-48cb-a9c9-894caa7db7af.png)
- 1138245-21-2
[(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate
| Conditions | Yield |
|---|---|
|
In water; Heating;
|
77% |
|
In tert-butyl methyl ether; water; acetone; at -10 - 20 ℃; for 4.5h; Reagent/catalyst; Solvent; Temperature; Time;
|
-
![diethyl [(1R,5S)-3-ethylbicyclo[3.2.0]hept-3-en-6-ylidene]propanedioate](/upload/2024/1/77528958-2977-4627-a21b-a0eac330fd7b.png)
-
diethyl [(1R,5S)-3-ethylbicyclo[3.2.0]hept-3-en-6-ylidene]propanedioate

-
![[(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate](/upload/2024/1/d20df947-c9cc-48cb-a9c9-894caa7db7af.png)
- 1138245-21-2
[(1R,5S,6S)-6-(Aminomethyl)-3-ethylbicyclo[3.2.0]hept-3-en-6-yl]acetic acid monobenzenesulfonate
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 4 steps
1.1: ethanol / 4 h / 20 °C
2.1: potassium hydroxide / ethanol; water / 10 h / 20 °C / Reflux
2.2: 10 - 20 °C
3.1: hydrogenchloride / water; tert-butyl methyl ether / 20 °C
3.3: sponge cobalt; dimethylpolysiloxane / 7.5 h / 40 °C / 3375.34 Torr
4.1: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With hydrogenchloride; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone;
|
|
|
Multi-step reaction with 4 steps
1.1: ethanol / 4 h / 20 °C
2.1: potassium hydroxide / ethanol / 7.5 h / Reflux
2.2: 2 h / 20 °C
2.3: 20 °C
3.1: hydrogenchloride / water; toluene
3.2: sponge nickel / 3 h / 20 °C / 3040.2 Torr
3.3: 0.5 h
4.1: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With hydrogenchloride; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone; toluene;
|
|
|
Multi-step reaction with 4 steps
1: ethanol / 4 h / 20 °C
2: potassium hydroxide / ethanol; water / 8 h / 26 - 27 °C / Reflux
3: ammonium hydroxide; hydrogen; potassium hydroxide / water / 15 h / 40 °C / 3000.3 Torr
4: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With ammonium hydroxide; hydrogen; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone;
|
|
|
Multi-step reaction with 5 steps
1.1: ethanol / 4 h / 20 °C
2.1: potassium hydroxide / ethanol; water / 8 h / 26 - 27 °C / Reflux
3.1: hydrogenchloride / toluene / 22 - 46 °C
4.1: hydrogenchloride / water; tert-butyl methyl ether / 20 °C
4.3: sponge cobalt; dimethylpolysiloxane / 7.5 h / 40 °C / 3375.34 Torr
5.1: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With hydrogenchloride; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone; toluene;
|
|
|
Multi-step reaction with 5 steps
1.1: ethanol / 4 h / 20 °C
2.1: potassium hydroxide / ethanol / 8 h / Reflux
2.2: 20 h / 20 °C
2.3: 20 °C
3.1: hydrogenchloride / water; toluene
4.1: ammonium hydroxide; hydrogen; potassium hydroxide / water / 15 h / 40 °C / 3000.3 Torr
5.1: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With hydrogenchloride; ammonium hydroxide; hydrogen; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone; toluene;
|
|
|
Multi-step reaction with 6 steps
1.1: ethanol / 4 h / 20 °C
2.1: potassium hydroxide / ethanol / 8 h / Reflux
2.2: 20 h / 20 °C
2.3: 20 °C
3.1: hydrogenchloride / water; toluene
4.1: hydrogenchloride / toluene / 22 - 46 °C
5.1: hydrogenchloride / water; tert-butyl methyl ether / 20 °C
5.3: sponge cobalt; dimethylpolysiloxane / 7.5 h / 40 °C / 3375.34 Torr
6.1: acetone; water; tert-butyl methyl ether / 4.5 h / -10 - 20 °C
With hydrogenchloride; potassium hydroxide; In ethanol; tert-butyl methyl ether; water; acetone; toluene;
|
1138245-21-2 Upstream products
-
1138245-13-2

DS-5565
-
98-11-3

benzenesulfonic acid
-
1235479-61-4
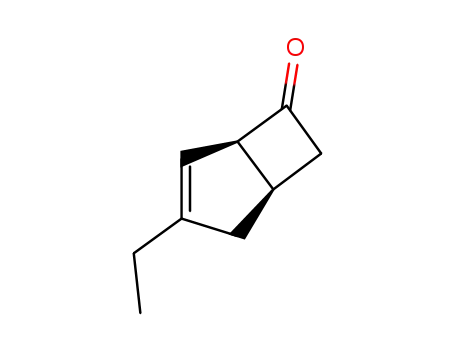
(1R,5S)-3-ethylbicyclo[3.2.0]hept-3-en-6-one
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Ibrutinib
CAS:936563-96-1
-
Cedazuridine
CAS:1141397-80-9