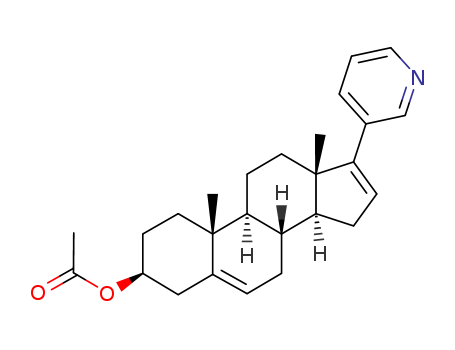
154229-18-2
- Product Name:Abiraterone Acetate
- Molecular Formula:C26H33NO2
- Purity:99%
- Molecular Weight:391.554
Product Details:
CasNo: 154229-18-2
Molecular Formula: C26H33NO2
Chinese Manufacturer Supply 154229-18-2, Sale Abiraterone Acetate In Bulk Supply
- Molecular Formula:C26H33NO2
- Molecular Weight:391.554
- Vapor Pressure:2.17E-10mmHg at 25°C
- Melting Point:127-130°C
- Refractive Index:1.583
- Boiling Point:506.709 °C at 760 mmHg
- PKA:5.31±0.12(Predicted)
- Flash Point:260.249 °C
- PSA:39.19000
- Density:1.14 g/cm3
- LogP:5.96940
Abiraterone Acetate(Cas 154229-18-2) Usage
|
Description |
Abiraterone acetate is an orally administered medication that acts as a selective inhibitor of CYP17, an enzyme crucial in androgen biosynthesis. By inhibiting this enzyme, abiraterone acetate blocks the production of androgens, which play a key role in the progression of prostate cancer from primary to metastatic stages, including the development of metastatic castration-resistant prostate cancer (mCRPC). |
|
Chemical Properties |
Off-White Solid |
|
Originator |
Institute of Cancer Research, London (United Kingdom) |
|
Uses |
Metastatic Castration-Resistant Prostate Cancer (mCRPC): Abiraterone acetate, in combination with a corticosteroid such as prednisone or methylprednisolone, is used for the treatment of metastatic castration-resistant prostate cancer. This includes patients whose cancer has not responded to treatments that lower testosterone levels and those who have previously received chemotherapy containing docetaxel. Metastatic High-Risk Castration-Sensitive Prostate Cancer: Abiraterone acetate, in combination with prednisone, is also approved for the treatment of metastatic high-risk castration-sensitive prostate cancer. This includes patients whose cancer has responded to treatments that lower testosterone levels. Mechanism of Action: Abiraterone acetate inhibits androgen biosynthesis by blocking the CYP17 enzyme, thereby reducing the production of androgens such as testosterone and dihydrotestosterone (DHT). This inhibition of androgen signaling helps slow the progression of prostate cancer and improve patient outcomes. |
|
Definition |
ChEBI: A sterol ester obtained by formal condensation of the 3-hydroxy group of abiraterone with the carboxy group of acetic acid. A prodrug that is converted in vivo to abiraterone. Used for treatment of metastatic castrate-resistant prostate cance . |
|
Brand name |
Zytiga |
|
Clinical Use |
Hormone antagonist: Treatment of metastatic prostate cancer |
InChI:InChI=1/C26H33NO2/c1-17(28)29-20-10-12-25(2)19(15-20)6-7-21-23-9-8-22(18-5-4-14-27-16-18)26(23,3)13-11-24(21)25/h4-6,8,14,16,20-21,23-24H,7,9-13,15H2,1-3H3/t20-,21?,23?,24?,25-,26+/m0/s1
154229-18-2 Relevant articles
Abiraterone Acetate: A Review in Metastatic Castration-Resistant Prostrate Cancer
Lesley J. Scott
, Drugs, Volume 77, pages 1565–1576, (2017)
Given its efficacy in prolonging OS and its convenient once-daily oral regimen, in combination with prednisone, abiraterone acetate is an important first-line option for the treatment of mCRPC.
Abiraterone acetate in the treatment of prostate cancer
Abhimanyu Thakur a 1, Aishwarya Roy a 2, Arijit Ghosh b 1, Mohit Chhabra c, Sugato Banerjee a
, Biomedicine & Pharmacotherapy Volume 101, May 2018, Pages 211-218
In this prevailing scenario, abiraterone acetate (AA) has proved to be a boon for patients suffering from prostate cancer. AA selectively inhibits the actions of enzymes C17, 20-lyase and 17α-hydroxylase on cytochrome P450 (CYP) 17 when administered orally.
Abiraterone acetate for castration resistant prostate cancer
Shreya Shah &Charles Ryan
, Expert Opinion on Investigational Drugs Volume 19, 2010 - Issue 4
One novel therapeutic is abiraterone acetate (AA): an inhibitor of CYP17, an enzyme that catalyzes two key serial reactions in androgen and estrogen biosynthesis. Data from Phase I and II trials suggest that clinically important antitumor activity is seen in up to 70% of castrate patients with advanced prostate cancer resistant to currently available endocrine therapies.
154229-18-2 Process route
-
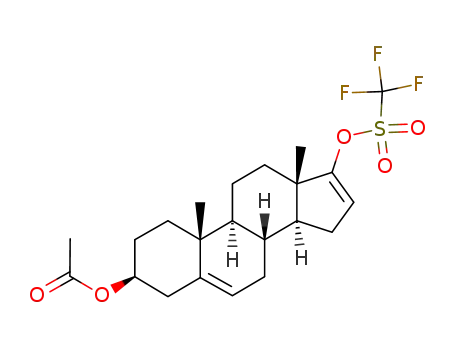
- 115375-60-5
3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate

-
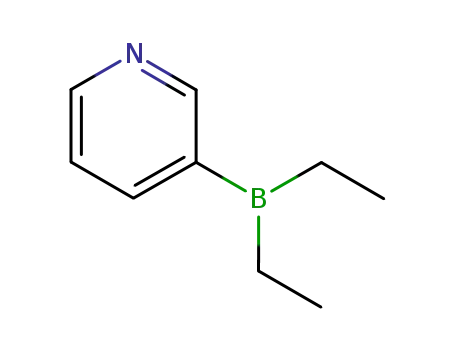
- 89878-14-8
3-Diethylboranylpyridine

-
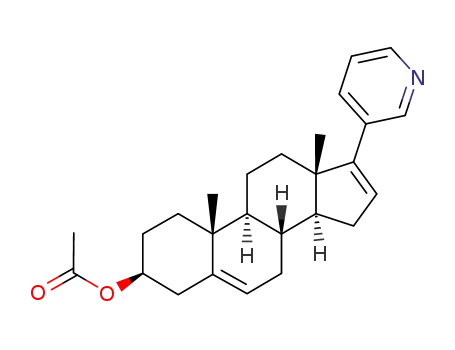
- 154229-18-2
abiraterone acetate
| Conditions | Yield |
|---|---|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; at 65 ℃; for 4h; Reflux;
|
95% |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; for 1h; Reflux;
|
95% |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium hydrogencarbonate; In tetrahydrofuran; water; at 60 ℃; for 1h;
|
94% |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; for 18h; Inert atmosphere; Reflux;
|
90% |
|
With bis(triphenylphosphine)palladium(II)-chloride; sodium carbonate; In tetrahydrofuran; at 80 ℃; for 1h;
|
84% |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium hydrogencarbonate; In tetrahydrofuran; water; at 60 ℃; for 23h; Inert atmosphere;
|
77% |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; for 1h; Inert atmosphere; Reflux;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In 2-methyltetrahydrofuran; water; at 20 - 80 ℃; for 2.15h; Inert atmosphere;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; at 66 ℃; for 3.5h;
|
15.68 g |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; at 25 - 80 ℃; for 6h; Inert atmosphere;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; at 20 ℃; for 1.5h; Temperature; Concentration; Reflux; Large scale;
|
65 kg |
|
3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate; 3-Diethylboranylpyridine; With bis-triphenylphosphine-palladium(II) chloride; In tetrahydrofuran; at 20 ℃; for 0.0833333h; Inert atmosphere;
With sodium carbonate; In tetrahydrofuran; water; Inert atmosphere; Reflux;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; Reflux; Inert atmosphere;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; for 2h; Inert atmosphere; Reflux;
|
|
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; water; for 2h; Reflux;
|
496.5 g |
|
With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate; In tetrahydrofuran; at 80 ℃;
|
-
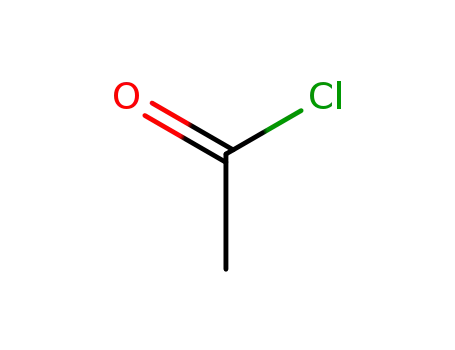
- 75-36-5
acetyl chloride

-
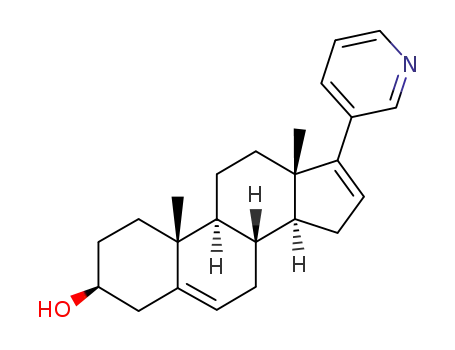
- 154229-19-3
abiraterone

-

- 154229-18-2
abiraterone acetate
| Conditions | Yield |
|---|---|
|
With N-ethyl-N,N-diisopropylamine; In diethyl ether; at 20 ℃; for 4h;
|
85.1% |
|
With 2-(Dimethylamino)pyridine; triethylamine; In diethyl ether; ethanol; hexane; water;
|
84% |
|
With triethylamine; In ethyl acetate; at 5 - 20 ℃; for 2h;
|
83% |
|
With N-ethyl-N,N-diisopropylamine; In diethyl ether; at 20 ℃; for 3h;
|
83.18% |
|
With triethylamine; In diethyl ether; at 20 ℃; for 3h; Concentration;
|
81.8% |
|
With pyridine; In dichloromethane;
|
70% |
|
With 2-(Dimethylamino)pyridine; In diethyl ether;
|
|
|
With dmap; triethylamine; In dichloromethane; at 20 - 30 ℃; for 2h;
|
0.745 g |
154229-18-2 Upstream products
-
115375-60-5

3β-acetoxyandrosta-5,16-dien-17-yl trifluoromethanesulphonate
-
89878-14-8

3-Diethylboranylpyridine
-
108-24-7
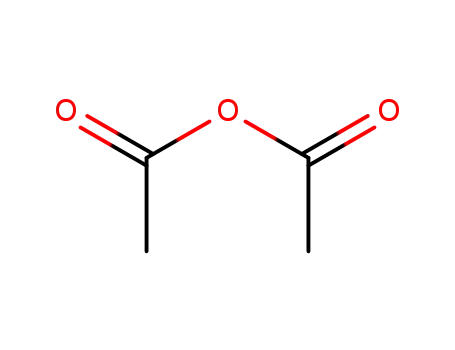
acetic anhydride
-
154229-19-3

abiraterone
154229-18-2 Downstream products
-
154229-19-3

abiraterone
-
154229-21-7
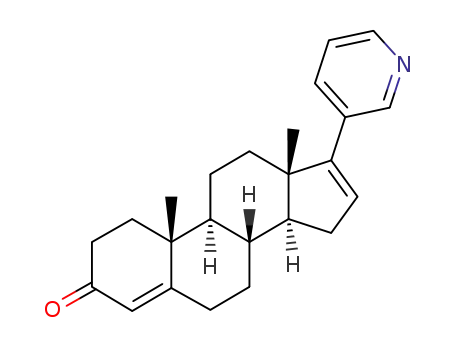
17-(3-pyridine)-4,16-dieneandrost-3-one
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Cedazuridine
CAS:1141397-80-9
-
Brexpiprazole
CAS:913611-97-9