132539-06-1
- Product Name:Olanzapine
- Molecular Formula:C17H20N4S
- Purity:99%
- Molecular Weight:312.439
Product Details:
CasNo: 132539-06-1
Molecular Formula: C17H20N4S
Appearance: yellowish crystalline powder
Buy High Grade Olanzapine 132539-06-1 Efficient Transportation
- Molecular Formula:C17H20N4S
- Molecular Weight:312.439
- Appearance/Colour:yellowish crystalline powder
- Vapor Pressure:0mmHg at 25°C
- Melting Point:195 °C
- Refractive Index:1.709
- Boiling Point:476.035 °C at 760 mmHg
- PKA:7.78±0.20(Predicted)
- Flash Point:241.697 °C
- PSA:59.11000
- Density:1.32 g/cm3
- LogP:2.88860
Olanzapine(Cas 132539-06-1) Usage
| Description |
Olanzapine is an atypical antipsychotic medication that acts by balancing the levels of neurotransmitters, particularly dopamine and serotonin, in the brain. It is sold under the brand name Zyprexa and is available in oral tablet form or as an injection administered into a muscle. |
|
Uses |
Schizophrenia: Olanzapine is used to treat the symptoms of schizophrenia, a mental illness characterized by disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions. It is effective for both new-onset disease and long-term maintenance therapy. Bipolar Disorder: Olanzapine may be used alone or in combination with other medications, such as lithium or valproate, to treat manic or mixed episodes associated with bipolar disorder (manic-depressive illness). It helps stabilize mood and reduce the intensity of manic or mixed symptoms. Mechanism of Action: Olanzapine acts by blocking the action of dopamine and serotonin receptors in the brain, thereby regulating neurotransmitter levels. By modulating dopamine and serotonin activity, it helps alleviate symptoms of psychosis, mania, and mood instability associated with schizophrenia and bipolar disorder. |
|
Chemical Properties |
Yellow Crystalline Powder |
|
Originator |
Lilly (USA) |
InChI:InChI=1/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3
132539-06-1 Relevant articles
Efficacy of Olanzapine and Olanzapine-Fluoxetine Combination in the Treatment of Bipolar I Depression
Mauricio Tohen, MD, DrPH; Eduard Vieta, MD, PhD; Joseph Calabrese, MD
, Arch Gen Psychiatry. 2003;60(11):1079-1088.
During all 8 study weeks, the olanzapine and olanzapine-fluoxetine groups showed statistically significant improvement in depressive symptoms vs the placebo group (P<.001 for all). The olanzapine-fluoxetine group also showed statistically greater improvement than the olanzapine group at weeks 4 through 8.
Direct Reductive Cyclocondensation of the Nitro Group with the Amido Group: Key Role of the Iminophosphorane Intermediate in the Synthesis of 1,4-Dibenzodiazepine Derivatives
Tryniszewski, Micha?,Bujok, Robert,Cmoch, Piotr,Gańczarczyk, Roman,Kulszewicz-Bajer, Irena,Wróbel, Zbigniew
, p. 2277 - 2286 (2019/05/16)
A class of dialkylamino-substituted dibe...
Olanzapine Versus Placebo in the Treatment of Acute Mania
Mauricio Tohen, M.D., Dr.P.H., Todd M. Sanger, Ph.D., Susan L. McElroy, M.D., Gary D. Tollefson, M.D., Ph.D., K.N.
American Journal of Psychiatry, 1999
Olanzapine, a recently introduced novel antipsychotic, demonstrates a pharmacologic profile that most closely resembles that of clozapine (19). In a series of double-blind controlled trials in patients with schizophrenia and schizoaffective disorder, olanzapine appeared superior to the conventional antipsychotic haloperidol on measures of overall psychotic symptoms, negative symptoms, comorbid mood symptoms, and quality of life...
132539-06-1 Process route
-
![2,4-bis(4-methyl-1-piperazinyl)-3-propylidene-3H-[1,5]benzodiazepine](/upload/2024/1/15f99068-110a-4b21-a98d-206850e08133.png)
-
2,4-bis(4-methyl-1-piperazinyl)-3-propylidene-3H-[1,5]benzodiazepine

-
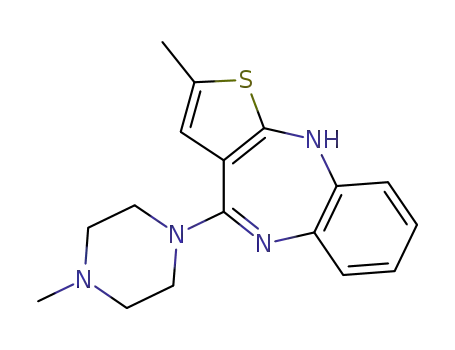
- 132539-06-1
zyprexa
| Conditions | Yield |
|---|---|
|
With quinoline p-toluenesulfonate; sulfur; In benzonitrile; at 140 ℃; for 13.5h;
|
69.5% |
|
With pyridinium p-toluenesulfonate; sulfur; In benzonitrile; at 140 ℃; for 13.5h;
|
66.6% |
|
With sulfur; 1-methyl-imidazolium p-toluenesulfonic acid; In benzonitrile; at 140 ℃; for 10h;
|
54.2% |
|
With toluene-4-sulfonic acid; sulfur; In benzonitrile; at 140 ℃; for 9h;
|
51.7% |
|
With sulfur; triethylamine; In propan-1-ol; dimethyl sulfoxide; at 100 ℃; for 120h;
|
12% |
-
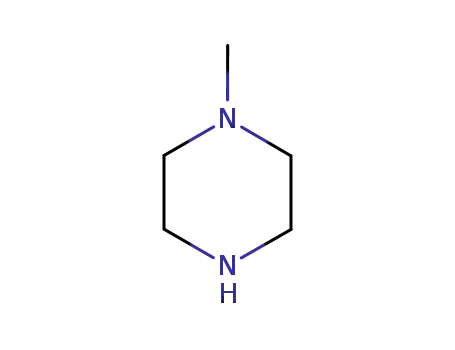
- 109-01-3
1-methyl-piperazine

-
![4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride](/upload/2024/1/5dc4e090-1188-4492-ac55-18dfdf3ab2fb.png)
- 138564-60-0
4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride

-

- 132539-06-1
zyprexa
| Conditions | Yield |
|---|---|
|
at 125 - 145 ℃; for 5 - 12h; Product distribution / selectivity; Neat (no solvent);
|
84% |
|
at 120 ℃; for 5h;
|
83.8% |
|
In dimethyl sulfoxide; at 112 - 115 ℃; for 16h;
|
82% |
|
1-methyl-piperazine; 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride; In dimethyl sulfoxide; at 112 - 115 ℃; for 16h;
With pyrographite; In dichloromethane; Product distribution / selectivity; Rapid crystallization;
|
82% |
|
1-methyl-piperazine; 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride; In dimethyl sulfoxide; at 112 - 115 ℃; for 16h;
In dichloromethane; Product distribution / selectivity; Rapid crystallization;
|
82% |
|
In 1,3-dimethyl-2-imidazolidinone; toluene; at 120 - 130 ℃; for 9 - 11h; Product distribution / selectivity;
|
76% |
|
1-methyl-piperazine; 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride; In dimethyl sulfoxide; at 112 - 115 ℃; for 16h;
With pyrographite; In methanol; dichloromethane; Product distribution / selectivity;
|
72% |
|
1-methyl-piperazine; 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride; In dimethyl sulfoxide; at 112 - 115 ℃; for 16h;
In methanol; dichloromethane; Product distribution / selectivity; Rapid crystallization;
|
72% |
|
In dimethyl sulfoxide; toluene; for 20h; Reflux; Inert atmosphere;
|
71% |
|
In dimethyl sulfoxide; toluene; for 24h; Heating;
|
65% |
|
In dimethyl sulfoxide; at 100 - 120 ℃; for 8h;
|
61% |
|
In dimethyl sulfoxide; at 138 ℃; for 22h; Product distribution / selectivity; Heating / reflux;
|
54% |
|
In acetonitrile; at 81 - 82 ℃; for 22h; Product distribution / selectivity; Heating / reflux;
|
53% |
|
In acetone; at 56 ℃; for 22 - 24h; Product distribution / selectivity; Heating / reflux;
|
51% |
|
In dimethyl sulfoxide; toluene; at 140 ℃; for 18h;
|
37% |
|
In dimethyl sulfoxide; toluene;
|
|
|
1-methyl-piperazine; 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride; In dimethyl sulfoxide; toluene; for 20h; Heating / reflux;
With ammonia; In dichloromethane; at 25 - 30 ℃; for 0.5h; Product distribution / selectivity; Heating / reflux;
|
|
|
at 120 ℃; for 3h; Product distribution / selectivity;
|
|
|
In dimethyl sulfoxide; toluene;
|
|
|
at 120 ℃; for 3h; Product distribution / selectivity;
|
|
|
In dimethyl sulfoxide; toluene; for 20h; Product distribution / selectivity; Heating / reflux;
|
|
|
In dimethyl sulfoxide; at 120 ℃; for 20h; Product distribution / selectivity;
|
|
|
at 160 ℃; for 0.5h; Sealed tube;
|
122 mg |
|
at 120 ℃; for 4h;
|
132539-06-1 Upstream products
-
109-01-3

1-methyl-piperazine
-
138564-60-0
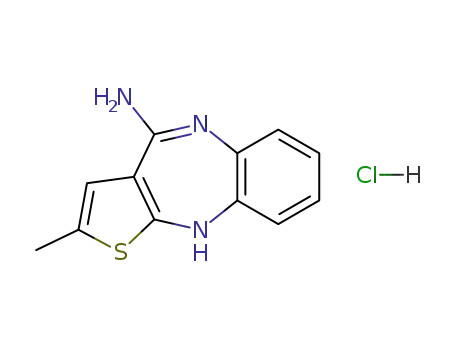
4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine hydrochloride
-
1493-27-2
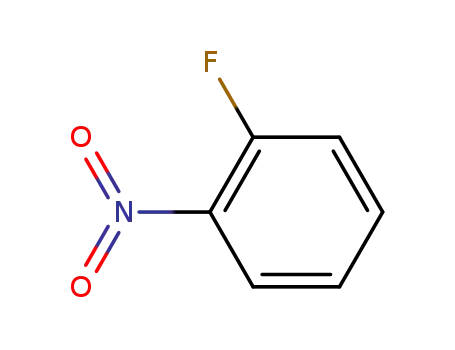
ortho-nitrofluorobenzene
-
138564-59-7
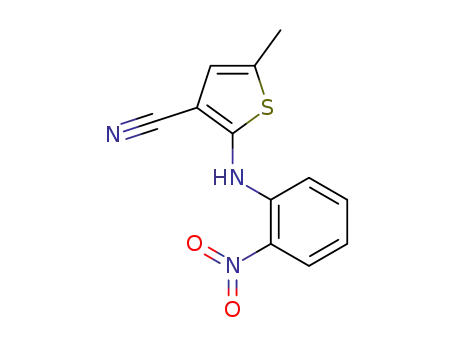
2-(2-nitroanilino)-5-methylthiophene-3-carbonitrile
132539-06-1 Downstream products
-
174794-02-6
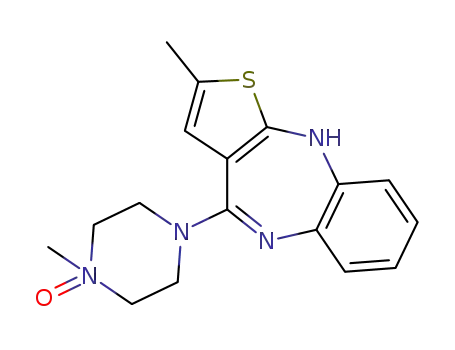
Olanzapine N-Oxide
-
221373-18-8
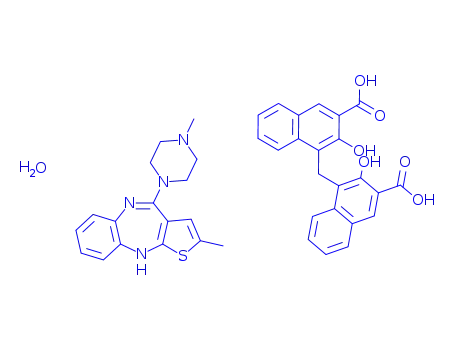
olanzapine pamoate monohydrate
-
929209-00-7
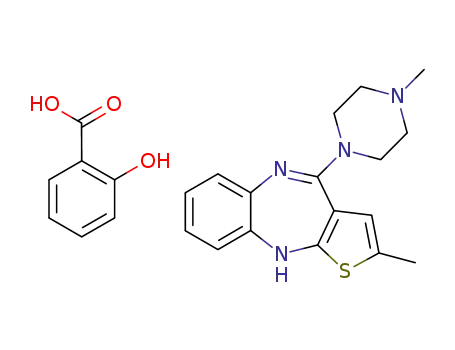
C7H6O3*C17H20N4S
-
1017241-34-7
C17H20N4O2
Relevant Products
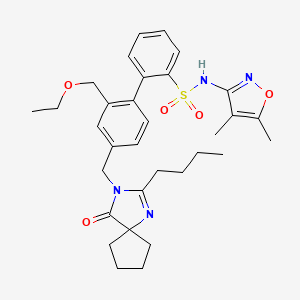
-
Sparsentan
CAS:254740-64-2
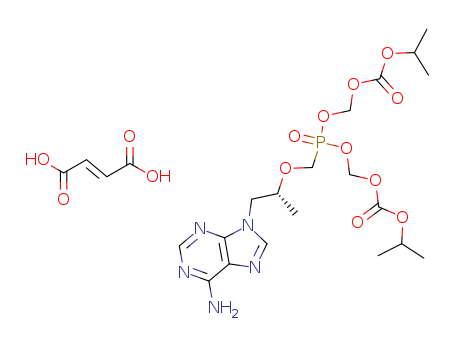
-
Tenofovir Disoproxil Fumarate
CAS:202138-50-9
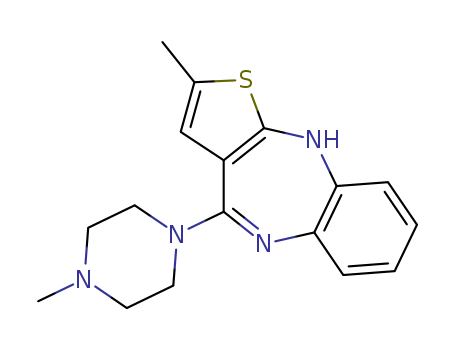
-
Capivasertib
CAS:1143532-39-1