202138-50-9
- Product Name:Tenofovir Disoproxil Fumarate
- Molecular Formula:C19H30N5O10P.C4H4O4
- Purity:99%
- Molecular Weight:635.522
Product Details:
CasNo: 202138-50-9
Molecular Formula: C19H30N5O10P.C4H4O4
Appearance: almost white crystalline
Buy High Quality Tenofovir Disoproxil Fumarate,Wholesale 202138-50-9 Safe Transportation
- Molecular Formula:C19H30N5O10P.C4H4O4
- Molecular Weight:635.522
- Appearance/Colour:almost white crystalline
- Vapor Pressure:2.06E-16mmHg at 25°C
- Melting Point:219oC
- Boiling Point:642.7 °C at 760 mmHg
- Flash Point:342.5 °C
- PSA:269.85000
- Density:1.45 g/cm3
- LogP:3.32860
Tenofovir disoproxil fumarate(Cas 202138-50-9) Usage
|
Overview |
Tenofovir Disoproxil Fumarate is an oral prodrug of tenofovir, which is a nucleotide analogue of adenosine monophosphate. Upon absorption, it is rapidly converted to its active form, tenofovir diphosphate, which inhibits the reverse transcriptase enzyme in retroviruses like HIV, preventing viral replication. It is sold under the brand name Viread. |
|
Uses |
Tenofovir Disoproxil Fumarate (TDF) belongs to the category of antiviral drugs. It is specifically classified as a nucleotide reverse transcriptase inhibitor (NRTI) used in the treatment of viral infections, particularly HIV and chronic hepatitis B virus (HBV) infections. After being metabolized intracellularly to tenofovir diphosphate, it acts as a competitive inhibitor of the HIV reverse transcriptase enzyme and causes DNA chain termination, effectively halting viral replication. Tenofovir Disoproxil Fumarate is used in combination with other antiretroviral medications to treat HIV-1 and HIV-2 infections. It is effective in controlling viral replication and improving patient outcomes by reducing viral load. |
| Stability | Tenofovir is not a substrate, inducer, or inhibitor of human cytochrome P450 enzymes. |
|
Brand name |
Viread (Gilead Sciences). |
| Dosage | The standard recommended dosage for adults is 300 mg/day. In patients with significant renal impairment, the dosage must be adjusted to avoid accumulation of the drug and associated toxicities. |
InChI:InChI=1/C19H30N5O10P.C4H4O4/c1-12(2)33-18(25)28-9-31-35(27,32-10-29-19(26)34-13(3)4)11-30-14(5)6-24-8-23-15-16(20)21-7-22-17(15)24;5-3(6)1-2-4(7)8/h7-8,12-14H,6,9-11H2,1-5H3,(H2,20,21,22);1-2H,(H,5,6)(H,7,8)/b;2-1+/t14-;/m1./s1
202138-50-9 Relevant articles
Tenofovir Disoproxil Fumarate Clinical Pharmacology and Pharmacokinetics
Brian P. Kearney, John F. Flaherty & Jaymin Shah
Clinical Pharmacokinetics, Volume 43, pages 595–612, (2004)
The recommended oral dosage of tenofovir DF in adults is 300 mg/day. Tenofovir is eliminated by renal elimination, including tubular secretion; dose-interval adjustments are necessary for tenofovir DF in patients with significant renal impairment. No dosage adjustment of tenofovir DF is necessary in patients with liver disease.
Tenofovir Disoproxil Fumarate Clinical Pharmacology and Pharmacokinetics
Brian P. Kearney, John F. Flaherty & Jaymin Shah
Clinical Pharmacokinetics, Volume 43, pages 595–612, (2004)
Tenofovir exhibits longer serum (17 hours) and intracellular (≥60 hours) half-lives than those of nucleoside analogues, which supports a flexible once-daily administration schedule. The pharmacokinetics of tenofovir are dose-proportional and similar in healthy volunteers and HIV-infected individuals.
The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years
Nelson, Mark Ra; Katlama, Christineb; Montaner, Julio Sc; Cooper, David Ad; Gazzard, Briana; Clotet, Bonaventurae; Lazzarin, Adrianof; Schewe, Knudg; Lange, Joeph; Wyatt, Christinai; Curtis, Suej; Chen, Shan-Shanj; Smith, Stephenj; Bischofberger, Norbertj; Rooney, James Fj for the Tenofovir DF Expanded Access Team
AIDS 21(10):p 1273-1281, June 2007.
To characterize the safety profile of tenofovir disoproxil fumarate (DF) for the treatment of HIV infection in adults over the first 4 years of use.
202138-50-9 Upstream products
-
110-17-8
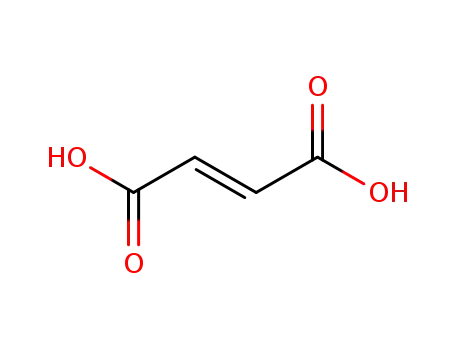
(2E)-but-2-enedioic acid
-
201341-05-1
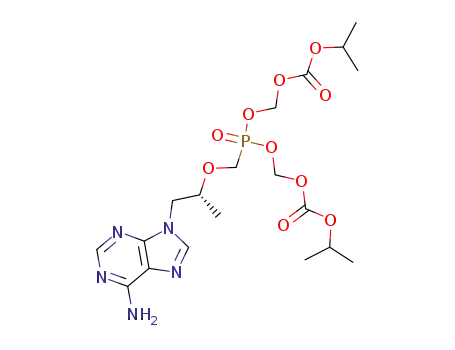
tenofovir disoproxil
-
66224-66-6
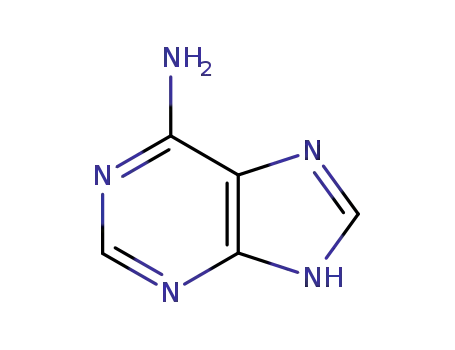
adenine
-
14047-28-0
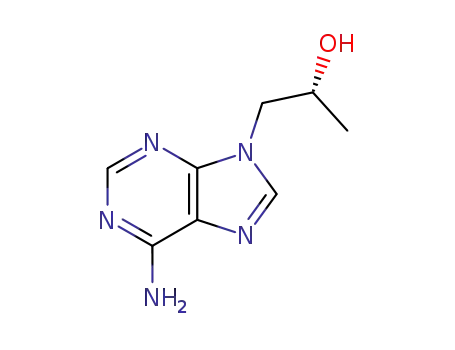
(R)-9-(2-hydroxypropyl)adenine
202138-50-9 Downstream products
-
1432630-26-6
9-[(R)-2-[[bis[[ (isopropoxycarbonyl)oxy]methoxy]phosphinyl]-methoxy]propyl]adenine fumarate Tenofovir disoproxil fumarate
-
201341-05-1

tenofovir disoproxil
Relevant Products
-
Fosfomycin tromethamine
CAS:78964-85-9
-
Lacosamide
CAS:175481-36-4
-
Olanzapine
CAS:132539-06-1