1143532-39-1
- Product Name:Capivasertib
- Molecular Formula:C21H25ClN6O2
- Purity:99%
- Molecular Weight:428.922
Product Details:
CasNo: 1143532-39-1
Molecular Formula: C21H25ClN6O2
Chinese Factory Supply High Purity 99% Capivasertib 1143532-39-1 Fast Shipping
- Molecular Formula:C21H25ClN6O2
- Molecular Weight:428.922
- PKA:13.93±0.50(Predicted)
- PSA:123.65000
- Density:1.381
- LogP:3.75450
Capivasertib(Cas 1143532-39-1) Usage
|
Description |
Capivasertib belongs to a class of medications called kinase inhibitors. Kinase inhibitors are drugs that target specific enzymes involved in cell signaling pathways, particularly those implicated in cancer cell growth and proliferation. Capivasertib specifically inhibits all isoforms of the AKT kinase, which plays a crucial role in promoting the growth and survival of cancer cells. |
| Uses |
Capivasertib is primarily used for the treatment of breast cancer, particularly in cases of advanced hormone receptor-positive (HR-positive) breast cancer. It is indicated for use in combination with another medication called fulvestrant (brand name Faslodex). The combination of capivasertib and fulvestrant has been shown to significantly reduce the risk of disease progression or death compared to fulvestrant alone. |
1143532-39-1 Relevant articles
SOLID STATE FORMS OF CAPIVASERTIB AND PROCESS FOR PREPARATION THEREOF
V Travani,DK Amec
WO2022147519A1
The present disclosure encompasses solid state forms of Capivasertib, in embodiments crystalline polymorphs, salts and co-crystals of Capivasertib, processes for preparation thereof, and pharmaceutical compositions thereof.
Abstract 1024: Combination activity of acalabrutinib and capivasertib in diffuse large B-cell lymphoma
K Burke,J Roderick-Richardson,N Narang,B Willis,A Bloecher
, Proceedings: AACR Annual Meeting 2021 April 10-15, 2021 and May 17-21, 2021 Philadelphia, PA 2021/07/01
Wide-ranging exploration of analogues of...
1143532-39-1 Process route
-
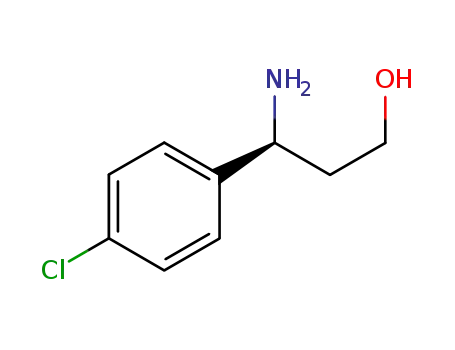
- 1213362-28-7,886061-26-3
(3S)-3-amino-3-(4-chlorophenyl)propan-1-ol

-
![8-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-2-oxa-4,8-diazaspiro[4.5]decane-1,3-dione](/upload/2024/1/37ed4ae0-aabd-4356-8d19-679b1f432c57.png)
-
8-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-2-oxa-4,8-diazaspiro[4.5]decane-1,3-dione

-
![4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxy-propyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide](/upload/2024/1/307aa9d1-4306-4503-984f-ada85f3e32e8.png)
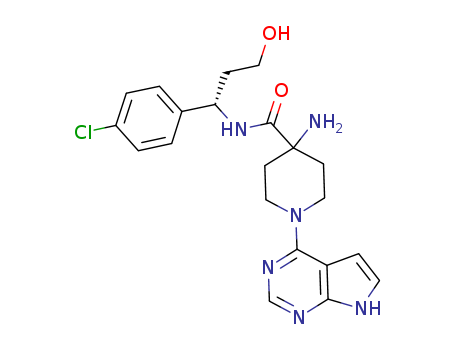
- 1143532-39-1
4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxy-propyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide
| Conditions | Yield |
|---|---|
|
With potassium hydrogencarbonate; In water; acetonitrile; at 25 ℃; for 10h;
|
80% |
-
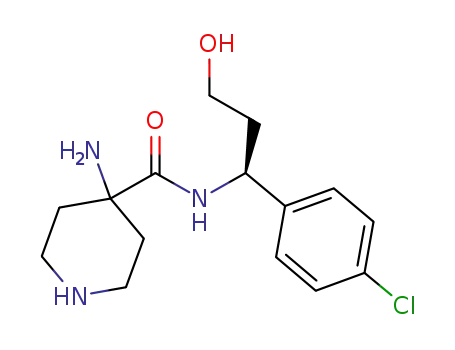
- 1143534-59-1
(S)-4-amino-N-(1-(4-chlorophenyl)-3-hydroxypropyl)piperidine-4-carboxamide

-
![4-chloro-1H-pyrrolo[2,3-d]pyrimidine](/upload/2024/1/b9b4009c-6f56-469d-a4b1-74fae0d2ca13.png)
- 3680-69-1
4-chloro-1H-pyrrolo[2,3-d]pyrimidine

-
![4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxy-propyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide](/upload/2024/1/307aa9d1-4306-4503-984f-ada85f3e32e8.png)
- 1143532-39-1
4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxy-propyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide
| Conditions | Yield |
|---|---|
|
With N-ethyl-N,N-diisopropylamine; In butan-1-ol; at 60 ℃; for 18h; Product distribution / selectivity;
|
42.5% |
1143532-39-1 Upstream products
-
1143534-59-1

(S)-4-amino-N-(1-(4-chlorophenyl)-3-hydroxypropyl)piperidine-4-carboxamide
-
3680-69-1
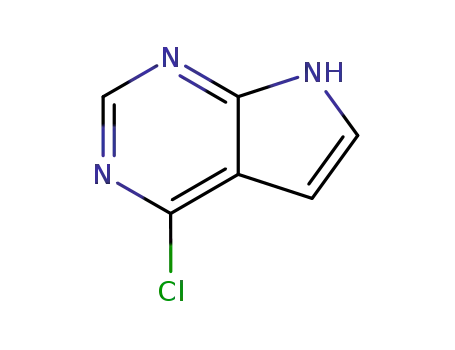
4-chloro-1H-pyrrolo[2,3-d]pyrimidine
-
1143534-12-6
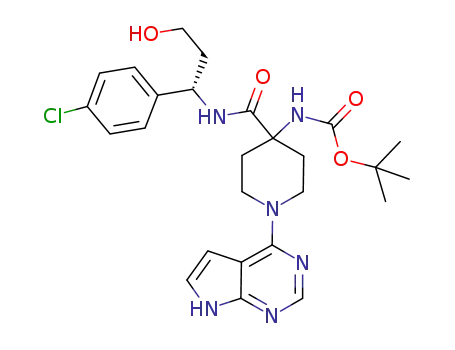
(S)-tert-butyl 4-(1-(4-chlorophenyl)-3-hydroxypropylcarbamoyl)-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidin-4-ylcarbamate
-
1213362-28-7

(3S)-3-amino-3-(4-chlorophenyl)propan-1-ol
Relevant Products
-
Milvexian
CAS:1802425-99-5
-
Olanzapine
CAS:132539-06-1
-
Ibrutinib
CAS:936563-96-1