125317-39-7
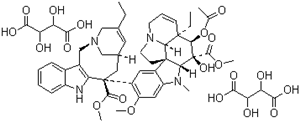
- Product Name:Vinorelbine Tartrate
- Molecular Formula:C45H54N4O8.2(C4H6O6)
- Purity:99%
- Molecular Weight:1079.11 .
Product Details:
CasNo: 125317-39-7
Molecular Formula: C45H54N4O8.2(C4H6O6)
Appearance: white to off-white crystalline powder
Factory Supply Best Quality Vinorelbine Tartrate 125317-39-7 Efficient Delivery
- Molecular Formula:C45H54N4O8.2(C4H6O6)
- Molecular Weight:1079.11 .
- Appearance/Colour:white to off-white crystalline powder
- Melting Point:181-183 °C
- Refractive Index:1.675
- PSA:363.99000
- Density:1.36 g/cm3
- LogP:0.44920
Vinorelbine tartrate(Cas 125317-39-7) Usage
|
Overview |
Vinorelbine Tartrate is a semi-synthetic vinca alkaloid used in the treatment of various cancers, primarily non-small cell lung cancer (NSCLC) and breast cancer. It works by disrupting the microtubule assembly, an essential process for cell division, thereby inhibiting cancer cell proliferation. Vinorelbine Tartrate is commonly administered intravenously as part of chemotherapy regimens. |
|
Chemical properties |
Appearance: White or pale yellow powder or crystalline powder Solubility: Soluble in water and slightly soluble in ethanol Stability: Stable under normal conditions but sensitive to light Vesicant: Vinorelbine tartrate is a moderate vesicant, meaning it can cause tissue damage at the injection site if extravasation occurs. |
| Mechanism of Action | Vinorelbine Tartrate inhibits mitosis by binding to tubulin, a protein required for the assembly of microtubules, which are essential for cell division. This leads to arrest of cell division at metaphase, preventing cancer cells from proliferating. Additionally, vinorelbine may affect several other cellular functions, including amino acid metabolism, ATPase activity, and nucleic acid biosynthesis, all of which contribute to its antitumor effects. |
|
Uses |
Vinorelbine Tartrate is indicated as a first-line therapy for advanced or metastatic NSCLC. It can be used alone or in combination with other chemotherapy agents like cisplatin or carboplatin to improve survival outcomes in patients with this condition.Vinorelbine Tartrate was approved by the U.S. Food and Drug Administration (FDA) in 1994 under the brand name Navelbine for the treatment of advanced NSCLC. It has since been approved for additional indications, including metastatic breast cancer. Ongoing studies are exploring its use in combination with newer therapies for expanded applications. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
|
Definition |
ChEBI: The L-(+)-tartrate salt of vinorelbine. |
InChI:InChI=1/C45H54N4O8/c1-8-27-19-28-22-44(40(51)55-6,36-30(25-48(23-27)24-28)29-13-10-11-14-33(29)46-36)32-20-31-34(21-35(32)54-5)47(4)38-43(31)16-18-49-17-12-15-42(9-2,37(43)49)39(57-26(3)50)45(38,53)41(52)56-7/h10-15,19-21,28,37-39,46,53H,8-9,16-18,22-25H2,1-7H3/t28-,37-,38+,39+,42+,43+,44-,45-/m0/s1
125317-39-7 Relevant articles
A Phase II trial of vinorelbine tartrate in patients with disseminated malignant melanoma and one prior systemic therapy
Robert P. Whitehead M.D., James Moon M.S., S. Spence McCachren M.D., Evan M. Hersh M.D., Wolfram E. Samlowski M.D., J. Thaddeus Beck M.D., Nerses S. Tchekmedyian M.D., Vernon K. Sondak M.D.
, Cancer, Volume100, Issue8 15 April 2004 Pages 1699-1704
There is a large population of patients who have progressed after first-line therapy and who might benefit from additional treatment. In the current study, patients who had failed first-line therapy were treated with the mitotic spindle inhibitor vinorelbine tartrate as a second-line agent to evaluate its antitumor effect and toxicity.
Vinorelbine tartrate (Navelbine): drug profile and nursing implications of a new vinca alkaloid.
Brogden JM 1 , Nevidjon B
, Oncology Nursing Forum, 01 May 1995, 22(4):635-646
To review the drug profile and nursing implications of a new vinca alkaloid, vinorelbine tartrate (Navelbine, Burroughs Wellcome Co., Research Triangle Park, NC).
Assessing and managing venous irritation associated with vinorelbine tartrate (Navelbine)
Rittenberg CN 1 , Gralla RJ , Rehmeyer TA
, Oncology Nursing Forum, 01 May 1995, 22(4):707-710
To determine the effect of duration of infusion time on venous irritation in patients receiving vinorelbine tartrate (Navelbine, Burroughs Wellcome Co., Research Triangle Park, NC) in combination with cisplatin or mitomycin.
125317-39-7 Downstream products
-
854607-08-2
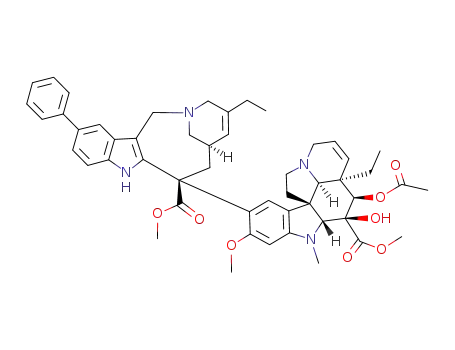
11'-phenylvinorelbine
-
854606-83-0
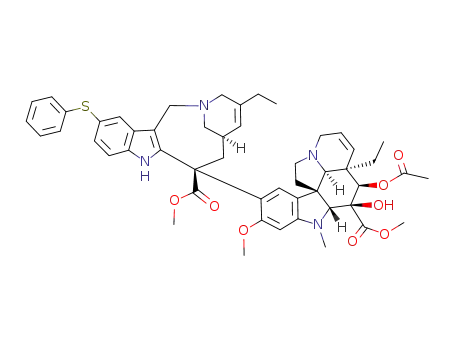
11'-(phenylsulfanyl)vinorelbine
-
854607-32-2
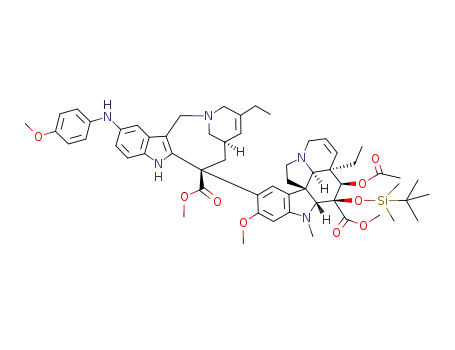
11'-(4-methoxyphenylamino)-3-(tert-butyldimethylsilanyloxy)vinorelbine
-
854607-29-7
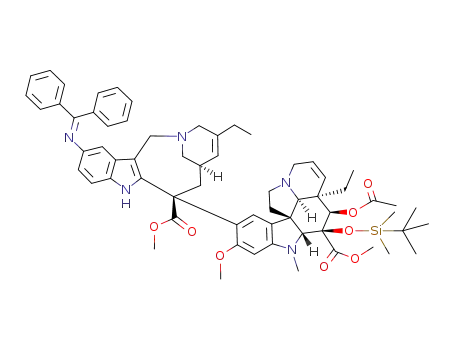
11'-benzhydrylidene-amino-3-(tert-butyldimethylsilanyloxy)vinorelbine
Relevant Products
-
Levofloxacin Hemihydrate
CAS:138199-71-0
-
Tenofovir Alafenamide Fumarate
CAS:1392275-56-7
-
Amikacin Sulphate
CAS:39831-55-5