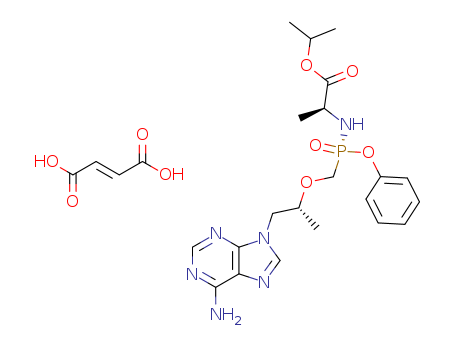
1392275-56-7
- Product Name:Tenofovir Alafenamide Fumarate
- Molecular Formula:2(C21H29N6O5P).C4H4O4
- Purity:99%
- Molecular Weight:592.546
Product Details:
CasNo: 1392275-56-7
Molecular Formula: 2(C21H29N6O5P).C4H4O4
Buy High Grade Top Purity Tenofovir Alafenamide Fumarate 1392275-56-7 Fast Delivery
- Molecular Formula:C4H4O4*C21H29N6O5P
- Molecular Weight:592.546
- PSA:381.18000
- LogP:7.60180
Tenofovir Alafenamide Fumarate(Cas 1392275-56-7) Usage
|
Description |
Tenofovir Alafenamide Fumarate (TAF) is a phosphonamide prodrug of tenofovir, primarily used to treat chronic Hepatitis B virus (HBV) infections and Human Immunodeficiency Virus (HIV) infections. TAF is metabolized intracellularly to its active form, tenofovir diphosphate, which inhibits the viral reverse transcriptase enzyme in both HBV and HIV, preventing viral replication. TAF is designed to be more effective and safer than its predecessor, Tenofovir Disoproxil Fumarate (TDF), with fewer side effects, particularly concerning kidney function and bone density. |
| Brand Names | Genvoya®: Combination product with elvitegravir, cobicistat, and emtricitabine. Odefsey®: Combination product with rilpivirine and emtricitabine. Descovy®: Combination product with emtricitabine. |
| Uses |
Antiretroviral (HIV): Used in combination with other agents as part of highly active antiretroviral therapy (HAART) to suppress viral replication in HIV-infected patients. Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Huarong Pharm adheres to our vision, our mission, and our value, keeping abreast of the current trend and state-of-the-art science and technologies of the global biopharmaceutical industry to serve our clients to the utmost satisfaction. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
| Pharmacokinetics | Absorption: Rapidly absorbed after oral administration, with preferential targeting of lymphatic tissues and liver. Metabolism: TAF is hydrolyzed intracellularly to tenofovir, which is then phosphorylated to the active form, tenofovir diphosphate. Excretion: Primarily excreted via the kidneys, though at much lower levels than TDF, reducing the risk of kidney-related side effects. |
1392275-56-7 Relevant articles
A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide
Chanie Wassner, PharmD, BCCCP, BCIDP https://orcid.org/0000-0003-3790-169X chanie.wassner@nyulangone.org, Nicole Bradley, PharmD, BCPS, BCIDP, and Yuman Lee, PharmD, BCIDP, AAHIVP
, Journal of the International Association of Providers of AIDS Care (JIAPAC)
Tenofovir is one of the newer, more tolerable, nucleotide reverse transcriptase inhibitors on the market; is a mainstay of many antiretroviral therapy combinations; and is now available in 2 different formulations, tenofovir disoproxil fumarate (TDF) and, the more recent, tenofovir alafenamide (TAF). These 2 formulations have very different pharmacokinetics, which seem to affect their efficacy and safety.
Tenofovir Alafenamide Vs. Tenofovir Disoproxil Fumarate in Single Tablet Regimens for Initial HIV-1 Therapy A Randomized Phase 2 Study
Sax, Paul E. MD*; Zolopa, Andrew MD†; Brar, Indira MD‡; Elion, Richard MD§; Ortiz, Roberto MD‖; Post, Frank MD, PhD, FCP (SA)¶; Wang, Hui PhD#; Callebaut, Christian PhD#; Martin, Hal MD, MPH#; Fordyce, Marshall W. MD#; McCallister, Scott MD#
, JAIDS Journal of Acquired Immune Deficiency Syndromes 67(1):p 52-58, September 1, 2014.
Antiretroviral naive adults with HIV-1 RNA ≥5000 copies per milliliter and a CD4 count ≥50 cells per microliter were randomized 2:1 to receive an STR of elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (E/C/F/TAF) or elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate (E/C/F/TDF), plus placebo for 48 weeks.
Tenofovir alafenamide versus tenofovir disoproxil fumarate: is there a true difference in efficacy and safety?
Andrew Hill 1, Sophie L. Hughes 2, Dzintars Gotham 2, Anton L. Pozniak 3
, Journal of Virus Eradication Volume 4, Issue 2, April 2018, Pages 72-79
The test for differences by boosting revealed substantial heterogeneity between boosted and unboosted subgroups for study discontinuations due to renal toxicity through week 48 (I2=73%). The risk of discontinuation for renal adverse events was 1% lower for boosted TAF than boosted TDF (95% CI -1% to 0%, P=0.002). However, there was no difference in the risk of discontinuation for renal adverse events for unboosted TAF and unboosted TDF (difference=0%, 95% CI 0%).
1392275-56-7 Upstream products
-
110-17-8
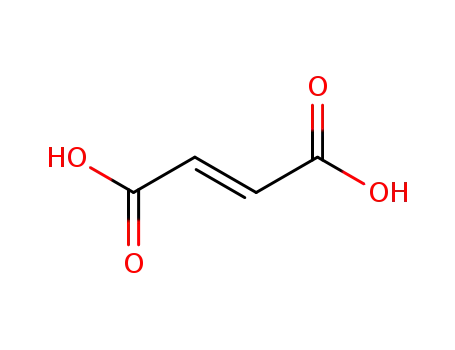
(2E)-but-2-enedioic acid
-
383365-04-6
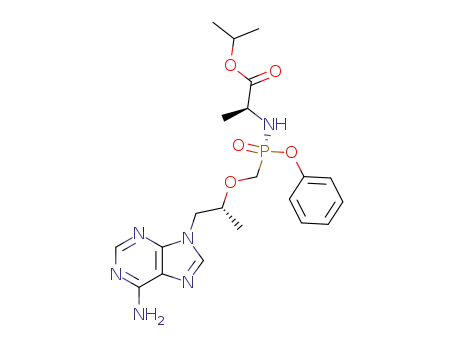
N-[(R)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphonyl]-L-alanine-1-methylethyl ester
-
39825-33-7
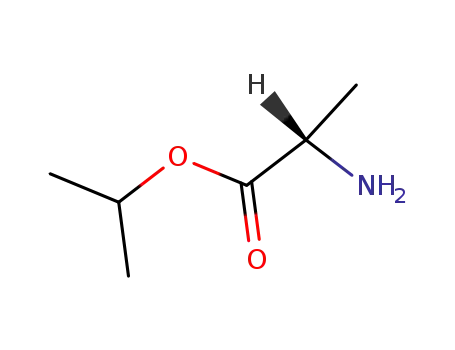
isopropyl L-alanine
-
147127-20-6
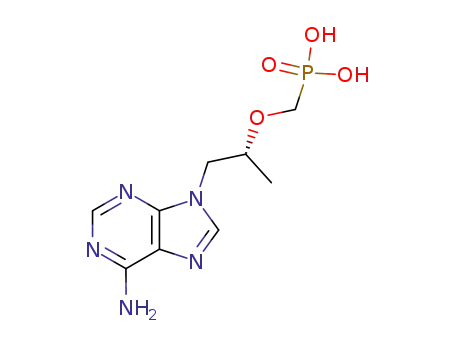
tenofovir
1392275-56-7 Downstream products
-
379270-37-8
(S)-isopropyl-2-(((S)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate
Relevant Products
-
Melamine Polyphosphate
CAS:218768-84-4
-
Gemfibrozil
CAS:25812-30-0
-
Vinorelbine Tartrate
CAS:125317-39-7