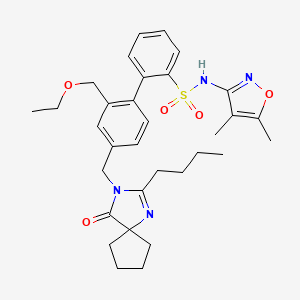
862189-95-5
- Product Name:Mirodenafil
- Molecular Formula:C26H37N5O5S
- Purity:99%
- Molecular Weight:531.67
Product Details:
CasNo: 862189-95-5
Molecular Formula: C26H37N5O5S
Quality Factory Supply Top Purity Mirodenafil 862189-95-5 Best Price
- Molecular Formula:C26H37N5O5S
- Molecular Weight:531.67
- Boiling Point:730.447 °C at 760 mmHg
- PKA:14.96±0.10(Predicted)
- Flash Point:395.56 °C
- PSA:129.14000
- Density:1.339 g/cm3
- LogP:3.40800
Mirodenafil(Cas 862189-95-5) Usage
|
Description |
Mirodenafil is a recently developed oral phosphodiesterase type 5 inhibitor (PDE5I) designed for treating erectile dysfunction (ED). As a second-generation PDE5I, it belongs to the drug class that includes well-known medications like avanafil, sildenafil, tadalafil, udenafil, and vardenafil, constituting the primary line of treatment for ED. Mirodenafil exhibits distinctive biochemical features, characterized by a notable affinity for PDE5 and high selectivity for this isoform over others. These properties distinguish it from existing PDE5Is such as sildenafil, vardenafil, and tadalafil, positioning Mirodenafil as a promising candidate for the management of erectile dysfunction. |
|
Consumer Uses |
ECHA has no public registered data indicating whether or in which chemical products the substance might be used. ECHA has no public registered data on the routes by which this substance is most likely to be released to the environment. |
| Factory | Hangzhou Huarong Pharm Co., Ltd. established since 2009 , has been always focusing on supplying products and services to our clients in the field of small molecule drug. Our existing advantages have led to our in-depth services for the R&D of small molecule drug discovery. |
862189-95-5 Relevant articles
Efficacy and Safety of Mirodenafil, A New Oral Phosphodiesterase Type 5 Inhibitor, for Treatment of Erectile Dysfunction
Jae‐Seung Paick, MD, PhD, Tai Y. Ahn, MD, PhD, Hyung K. Choi, MD, PhD, Woo‐Sik Chung, MD, PhD, Je J. Kim, MD, PhD, Sae C. Kim, MD, PhD, Sae W. Kim, MD, PhD, Sung W. Lee, MD, PhD, Kweon S. Min, MD, PhD, Ki H. Moon, MD, PhD ...
The Journal of Sexual Medicine, Volume 5, Issue 11, November 2008, Pages 2672–2680
A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, fixed‐dose study was conducted with 223 subjects who were randomized to placebo or mirodenafil at fixed doses of 50 or 100 mg for 12 weeks on an “as needed” basis.
Dose-dependent pharmacokinetics and first-pass effects of mirodenafil, a new erectogenic, in rats
Young H. Choi, Young S. Lee, Soo H. Bae, Tae K. Kim, Bong-Y. Lee, Myung G. Lee
, Biopharmaceutics & Drug Disposition, Volume30, Issue6 September 2009 Pages 305-317
The pharmacokinetics of mirodenafil and SK3541 were dose-dependent after both intravenous and oral administration of mirodenafil due to the saturable hepatic metabolism of mirodenafil.
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
Rivaroxaban intermediate
CAS:446292-08-6
-
Theophylline Anhydrous
CAS:58-55-9