1206123-37-6
- Product Name:Etrasimod
- Molecular Formula:C26H26F3NO3
- Purity:99%
- Molecular Weight:457.493
Product Details:
CasNo: 1206123-37-6
Molecular Formula: C26H26F3NO3
Factory Supply High Purity Etrasimod ,Offer 1206123-37-6 Cheapest Price
- Molecular Formula:C26H26F3NO3
- Molecular Weight:457.493
- Boiling Point:621.4±50.0 °C(Predicted)
- PKA:4.61±0.10(Predicted)
- PSA:62.32000
- Density:1.326±0.06 g/cm3(Predicted)
- LogP:6.92780
Etrasimod(Cas 1206123-37-6) Usage
|
Description |
Etrasimod is a selective sphingosine-1-phosphate (S1P) receptor modulator, available in oral tablet form. It belongs to the class of medications known as sphingosine 1-phosphate receptor modulators, which act on the immune system to modify its activity. By selectively binding to S1P receptor subtypes 1, 4, and 5, etrasimod modifies immune system activity, addressing inflammation and symptoms associated with ulcerative colitis. |
|
Uses |
Etrasimod is indicated for the treatment of moderate to severe active ulcerative colitis, a condition characterized by inflammation and sores in the colon and rectum. It is prescribed for adults who have not responded adequately to other therapies for ulcerative colitis. Etrasimod provides an option for individuals with this condition to manage symptoms effectively and improve quality of life. |
1206123-37-6 Relevant articles
Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies
WJ Sandborn,S Vermeire,L Peyrin-Biroulet,MC Dubinsky,J Panes,A Yarur,T Ritter,F Baert,S Schreiber,S Sloan
The Lancet, 2023
In two independent randomised, multicentre, double-blind, placebo-controlled, phase 3 trials, ELEVATE UC 52 and ELEVATE UC 12, adults with active moderate-to-severe ulcerative colitis and an inadequate or loss of response or intolerance to at least one approved ulcerative colitis therapy were randomly assigned (2:1) to once-daily oral etrasimod 2 mg or placebo. Patients in ELEVATE UC 52 were enrolled from 315 centres in 40 countries.
Discovery of APD334: Design of a clinical stage functional antagonist of the sphingosine-1-phosphate-1 receptor
Buzard, Daniel J.,Kim, Sun Hee,Lopez, Luis,Kawasaki, Andrew,Zhu, Xiuwen,Moody, Jeanne,Thoresen, Lars,Calderon, Imelda,Ullman, Brett,Han, Sangdon,Lehmann, Juerg,Gharbaoui, Tawfik,Sengupta, Dipanjan,Calvano, Lorene,Montalban, Antonio Garrido,Ma, You-An,Sage, Carleton,Gao, Yinghong,Semple, Graeme,Edwards, Jeff,Barden, Jeremy,Morgan, Michael,Chen, Weichao,Usmani, Khawja,Chen, Chuan,Sadeque, Abu,Christopher, Ronald J.,Thatte, Jayant,Fu, Lixia,Solomon, Michelle,Mills, David,Whelan, Kevin,Al-Shamma, Hussien,Gatlin, Joel,Le, Minh,Gaidarov, Ibragim,Anthony, Todd,Unett, David J.,Blackburn, Anthony,Rueter, Jaimie,Stirn, Scott,Behan, Dominic P.,Jones, Robert M.
, p. 1313 - 1317 (2015/01/09)
APD334 was discovered as part of our int...
A Randomized, Double-Blind, Placebo-Controlled Trial of a Selective, Oral Sphingosine 1-Phosphate (S1P) Receptor Modulator, Etrasimod (APD334), in Moderate to Severe Ulcerative Colitis (UC): Results From the OASIS Study: ACG Auxiliary Award (Member) 569
Sandborn, William J. MD, FACG1; Peyrin-Biroulet, Laurent MD, PhD2; Trokan, Luba3; Zhang, Jinkun3; Kühbacher, Tanja MD, PhD4; Chiorean, Michael MD5; Lee, Scott D. MD6; Vermeire, Severine MD, PhD7; Yacyshyn, Bruce MD, FACG8; Naik, Snehal3; Klassen, Preston3; Panes, Julian PhD9
, American Journal of Gastroenterology 113():p S327-S328, October 2018.
This randomized, double-blind, parallel-group, 12-week (wk) phase 2 induction study evaluated etrasimod in pts with moderate-severe UC, defined as 3-component Mayo Clinic Score (MCS) of 4-9 with endoscopic subscore ≥2 and rectal bleeding (RB) subscore ≥1. The 3-component MCS (range 0-9) includes RB, stool frequency, and endoscopy.
1206123-37-6 Process route
-
![(R/S)-ethyl 2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate](/upload/2024/1/e74528e9-7504-41ff-89d6-a4dcac01b7c7.png)
- 1206124-34-6
(R/S)-ethyl 2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate

-
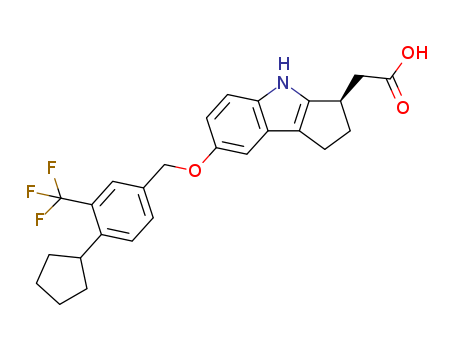
![[(3R)-7-{[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy}-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid](/upload/2024/1/4a18cd1e-5240-4165-9885-55670493f44a.png)
- 1206123-37-6
[(3R)-7-{[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy}-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid
| Conditions | Yield |
|---|---|
|
(R/S)-ethyl 2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetate; With water; recombinant yeast Lipase B Candida antarctica immobilized on Immobead 150; In acetonitrile; at 40 ℃; for 16h; pH=7.8; Inert atmosphere; Enzymatic reaction; Aqueous phosphate buffer;
With citric acid; In water; acetonitrile; pH=3.96; Product distribution / selectivity;
|
99.42 % ee |
|
With Candida antarctica lipase B; In aq. phosphate buffer; acetonitrile; Inert atmosphere; Large scale; Enzymatic reaction;
|
1.318 kg |
-
![2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid](/upload/2024/1/86f4f515-6967-4aa9-b094-26379434d054.png)
- 1206123-55-8
2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid

-
![(S)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid](/upload/2024/1/8b4c6615-7cc8-4f2e-abbe-5905a9ef8304.png)
- 1206123-51-4
(S)-2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid

-
![[(3R)-7-{[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy}-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid](/upload/2024/1/4a18cd1e-5240-4165-9885-55670493f44a.png)
- 1206123-37-6
[(3R)-7-{[4-cyclopentyl-3-(trifluoromethyl)phenyl]methoxy}-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl]acetic acid
| Conditions | Yield |
|---|---|
|
With trifluoroacetic acid; In hexane; isopropyl alcohol; Resolution of racemate;
|
1206123-37-6 Upstream products
-
1206123-55-8
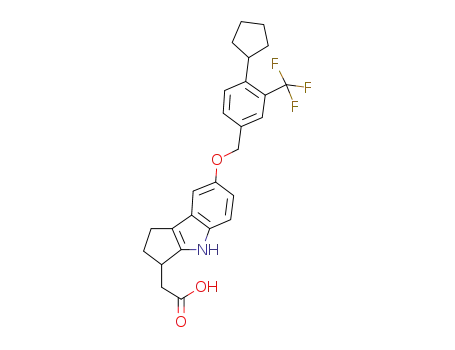
2-(7-(4-cyclopentyl-3-(trifluoromethyl)benzyloxy)-1,2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetic acid
-
120-92-3
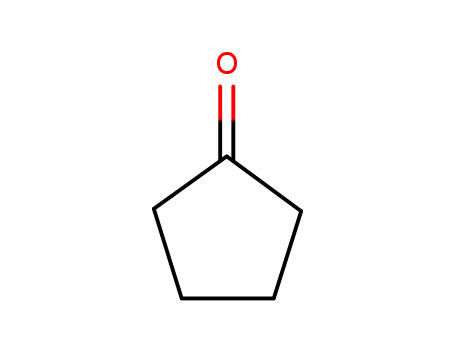
cyclopentanone
-
115591-64-5
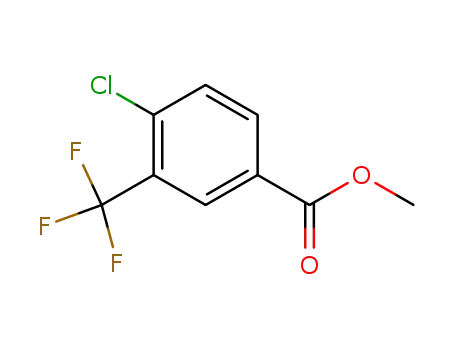
4-chloro-3-trifluoromethylbenzoic acid methyl ester
-
611-10-9
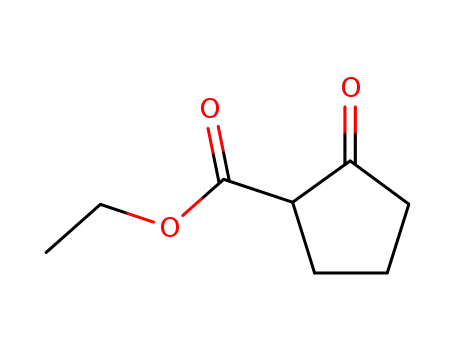
2-ethoxycarbonyl-1-cyclopentanone
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Apremilast
CAS:608141-41-9
-
Rimegepant
CAS:1289023-67-1