608141-41-9
- Product Name:Apremilast
- Molecular Formula:C22H24N2O7S
- Purity:99%
- Molecular Weight:460.508
Product Details:
CasNo: 608141-41-9
Molecular Formula: C22H24N2O7S
Factory Supply High Purity 99% Pure Apremilast 608141-41-9 Safe Shipping
- Molecular Formula:C22H24N2O7S
- Molecular Weight:460.508
- Boiling Point:741.342 °C at 760 mmHg
- PKA:14.01±0.20(Predicted)
- Flash Point:402.149 °C
- PSA:127.46000
- Density:1.382 g/cm3
- LogP:3.52590
ApreMilast(Cas 608141-41-9) Usage
|
Description |
Apremilast, marketed under the brand name Otezla, is a medication approved by the FDA for the treatment of certain types of psoriasis, psoriatic arthritis, and ulcers in the mouth associated with Behcet's syndrome. It belongs to a class of oral small molecule inhibitors known as phosphodiesterase 4 (PDE4) inhibitors. |
| Indications |
Apremilast is used for the treatment of active psoriatic arthritis in adults. It is also employed for the management of moderate to severe plaque psoriasis in certain patients. |
| Uses |
Apremilast is indicated for the treatment of adult patients with active psoriatic arthritis. Apremilast helps improve skin symptoms, including redness, scaling, and thickness caused by plaque psoriasis. |
| FDA Approval | Apremilast received FDA approval on March 21, 2014, for the treatment of adult patients with active psoriatic arthritis. It is also approved for use in adults with plaque psoriasis who are candidates for phototherapy or systemic therapy, as well as adults with Behcet's disease associated with oral ulcers. |
InChI:N-{2-[(1S)-1-(3-Ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}acetamide
608141-41-9 Relevant articles
Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial
Dr Kim Papp, MD Jennifer C Cather, MD Les Rosoph, MD Howard Sofen, MD Richard G Langley, MD Robert T Matheson, MD
, The Lancet, VOLUME 380, ISSUE 9843, P738-746, AUGUST 25, 2012
At week 16, patients in the placebo group were assigned apremilast 20 or 30 mg twice daily until week 24. Randomisation was generated with a permuted-block randomisation list via interactive voice response system. For the first 16 weeks, treatment assignment was concealed from both investigators and participants.
Chemoenzymatic synthesis of apremilast: A study using ketoreductases and lipases
Vega, Kimberly B.,Cruz, Daniel M. V.,Oliveira, Artur R. T.,Da Silva, Marcos R.,De Lemos, Telma L. G.,Oliveira, Maria C. F.,Bernardo, Ricardo D. S.,De Sousa, Jackson R.,Zanatta, Geancarlo,Nasário, Fábio D.,Marsaioli, Anita J.,De Mattos, Marcos C.
, p. 1100 - 1110 (2021/05/19)
The key step in the chemoenzymatic synth...
Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in ankylosing spondylitis
Ejaz Pathan1, Sonya Abraham2, Elizabeth Van Rossen3, Robin Withrington3, Andrew Keat4, Peter J Charles1, Erin Paterson1, Muslima Chowdhury1, Catherine McClinton5, Peter C Taylor5
Annals of the Rheumatic Diseases, Volume 72, Issue 9
In this double-blind, placebo-controlled, single-centre, Phase II study, patients with symptomatic AS with active disease on MRI were randomised to apremilast 30 mg BID or placebo over 12 weeks. Bath Indices were monitored serially. Patients were followed for 4 weeks after stopping medication. Bone biomarkers were assessed at baseline and day 85.
608141-41-9 Upstream products
-
6296-53-3
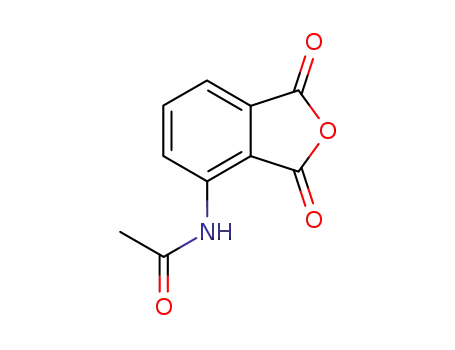
3-acetylaminophthalic anhydride
-
608141-43-1
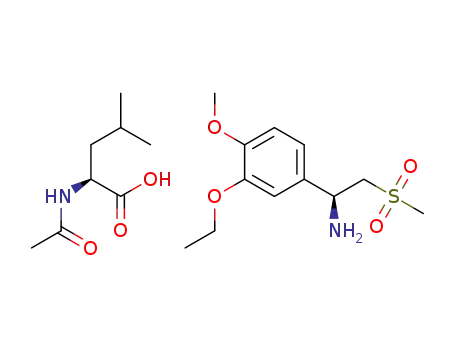
(S)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine-(S)-2-acetamido-4-methylpentanoate
-
608141-42-0
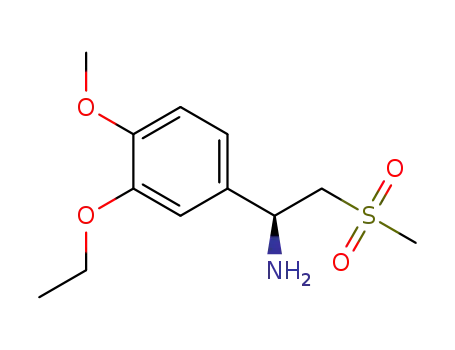
(S)-2-[1 (3-ethoxy-4-methoxyphenyl)]-1-methanesulfonyl-2-ethylamine
-
1450657-31-4
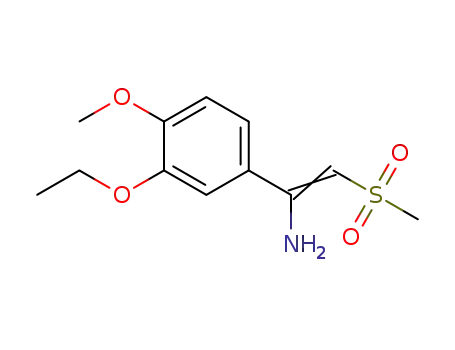
1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethen-1-amine
608141-41-9 Downstream products
-
1384967-20-7
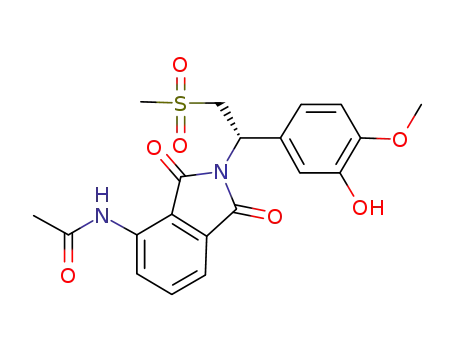
(S)-N-{2-[1-(3-hydroxy-4-methoxy-phenyl)-2-methanesulfonyl-ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}-acetamide
-
1384441-38-6
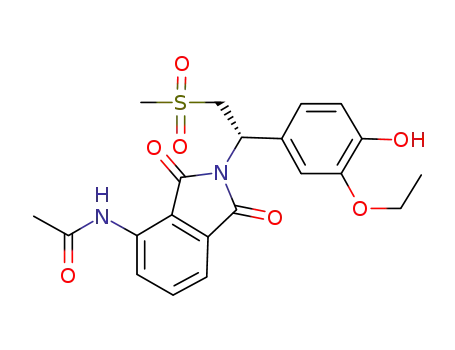
(S)-N-(2-(1-(3-ethoxy-4-hydroxyphenyl)-2-(methylsulfonyl)ethyl)-1,3-dioxoisoindolin-4-yl)acetamide
-
1384439-79-5
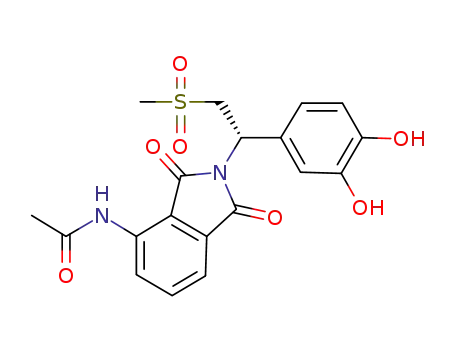
(S)-N-{2-[1-(3,4-dihydroxy-phenyl)-2-methanesulfonyl-ethyl]-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl}-acetamide
-
1384967-21-8
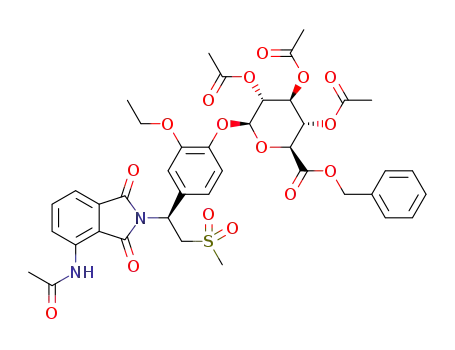
(2S,3R,4S,5S,6S)-2-(4-((S)-1-(4-acetamido-1,3-dioxoisoindolin-2-yl)-2-(methylsulfonyl)ethyl)-2-ethoxyphenoxy)-6-((benzyloxy)carbonyl)tetrahydro-2H-pyran-3,4,5-triyltriacetate
Relevant Products
-
Sparsentan
CAS:254740-64-2
-
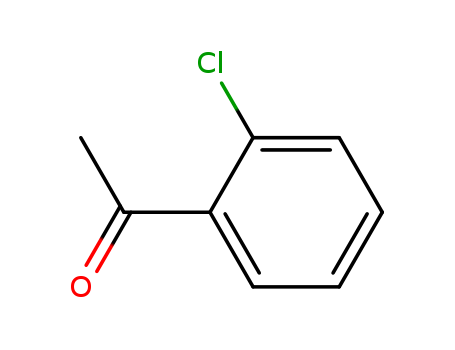
2'-Chloro acetophenone
CAS:2142-68-9
-
Etrasimod
CAS:1206123-37-6