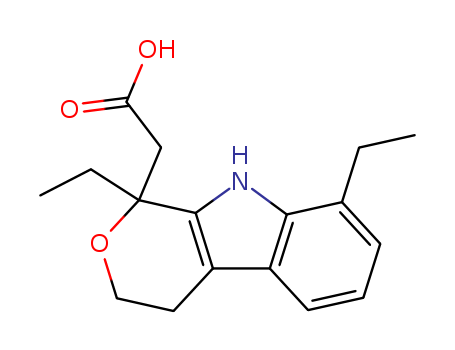
41340-25-4
- Product Name:Etodolac
- Molecular Formula:C17H21NO3
- Purity:99%
- Molecular Weight:287.359
Product Details:
CasNo: 41340-25-4
Molecular Formula: C17H21NO3
Appearance: crystalline solid
Buy High Grade Reliable Quality Etodolac 41340-25-4 In Bulk Supply
- Molecular Formula:C17H21NO3
- Molecular Weight:287.359
- Appearance/Colour:crystalline solid
- Melting Point:145-148 °C
- Boiling Point:507.9 °C at 760 mmHg
- PKA:4.65(at 25℃)
- Flash Point:260.9 °C
- PSA:62.32000
- Density:1.193 g/cm3
- LogP:3.38300
Etodolac(Cas 41340-25-4) Usage
| Description | Etodolac belongs to the class of nonsteroidal anti-inflammatory drugs (NSAIDs) and is used primarily for its analgesic (pain-relieving) and anti-inflammatory effects. It is classified as a pyranocarboxylic acid, representing a new chemical class of anti-inflammatory drugs. Etodolac works by reducing hormones in the body that cause inflammation and pain, making it effective in managing various painful conditions, including arthritis. |
|
Uses |
Etodolac is indicated for the treatment of mild to moderate pain and inflammation associated with various conditions, including: Arthritis: Etodolac is used to relieve symptoms of arthritis, including osteoarthritis and rheumatoid arthritis. It helps reduce inflammation, swelling, stiffness, and joint pain associated with these conditions. Postoperative pain: Etodolac is also used in the clinical treatment of postoperative pain, providing relief from pain and inflammation following surgical procedures. Other painful conditions: Etodolac may be prescribed for other painful conditions where inflammation is a contributing factor, such as musculoskeletal injuries, dental pain, and menstrual cramps. |
|
Originator |
Ayerst (USA) |
|
Brand name |
Lodine(Wyeth). |
InChI:InChI=1/C17H21NO3/c1-3-11-6-5-7-12-13-8-9-21-17(4-2,10-14(19)20)16(13)18-15(11)12/h5-7,18H,3-4,8-10H2,1-2H3,(H,19,20)
41340-25-4 Relevant articles
Double-blind, parallel comparison of etodolac and naproxen in patients with osteoarthritis of the knee
T.G. Palferman,G.R. Struthers,P.I. Williams
, acta therapeutica, 1991
Patients in both treatment groups showed prompt response to therapy. The etodolac group had significant improvement from baseline for 10 efficacy variables at week 2, 12 variables at week 4, 13 variables at week 6, and 14 variables at final evaluation. In comparison, the naproxen treatment group showed significant improvement in 13 variables at week 2, 11 at week 4, and 10 at week 6 and the final evaluation. The patient's global evaluation indicated that 69% of the etodolac-treated patients had improvement in their condition...
A review of the antiarthritic efficacy and safety of etodolac
N. Zvaifler M.D.
Clinical Rheumatology, Volume 8, pages 43–53, (1989)
Gastrointestinal microbleeding associated with etodolac was comparable to that with placebo and was significantly less than that associated with other commonly used NSAIDs, such as ibuprofen, indomethacin, piroxicam, and naproxen.
Effectiveness of etodolac (‘Lodine’) compared with naproxen in patients with acute gout
Armando Maccagno,Eduardo Di Giorgio &Alberto Romanowicz
, Current Medical Research and Opinion Volume 12, 1991 - Issue 7
Patients were allocated at random to receive either 300 mg etodolac twice daily (31 patients) or 500 mg naproxen twice daily (30 patients) for 7 days. Both groups were comparable for sex, age and weight of patients, but there was a tendency for patients in the etodolac group to have more severe gout as shown by baseline clinical assessment scores.
41340-25-4 Process route
-
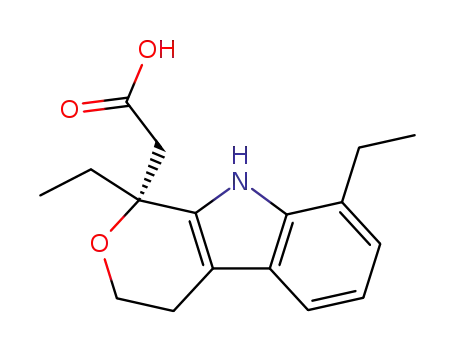
- 87226-41-3
(R)-etodolac

-
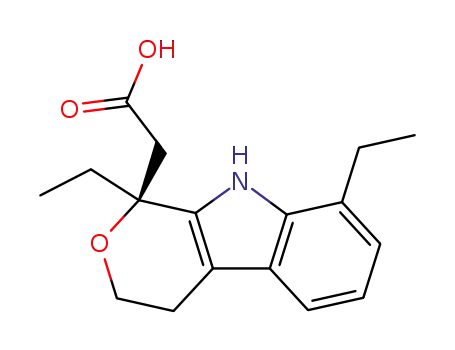
- 87249-11-4
(S)-etodolac

-
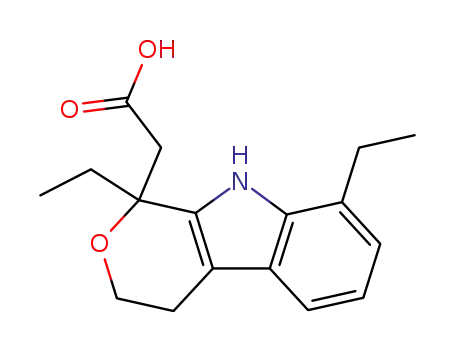
- 41340-25-4
etodolac
| Conditions | Yield |
|---|---|
|
With tin(IV) chloride; In dichloromethane; at 20 ℃; for 2h;
|
|
|
(R)-etodolac; (S)-etodolac; In dichloromethane; at 20 ℃; for 2h;
With sodium hydroxide; formic acid; at 0 ℃; for 1h; pH=5;
|
-

- 87226-41-3
(R)-etodolac

-

- 41340-25-4
etodolac
| Conditions | Yield |
|---|---|
|
With tin(IV) chloride; In tetrahydrofuran; dichloromethane; for 4h; Heating / reflux;
|
|
|
sulfuric acid; In isopropyl alcohol; at 20 ℃; for 23h; Product distribution / selectivity;
|
41340-25-4 Upstream products
-
41340-36-7
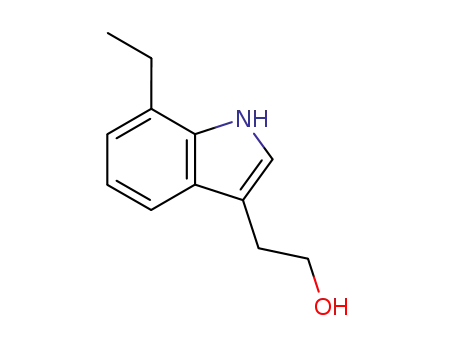
7-ethyl-2-(1H-indol-3-yl)ethan-1-ol
-
4949-44-4
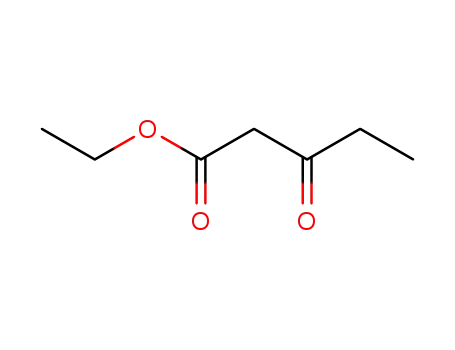
ethyl propanoylacetate
-
87226-41-3

(R)-etodolac
-
87249-11-4

(S)-etodolac
41340-25-4 Downstream products
-
41340-36-7

7-ethyl-2-(1H-indol-3-yl)ethan-1-ol
-
115066-03-0
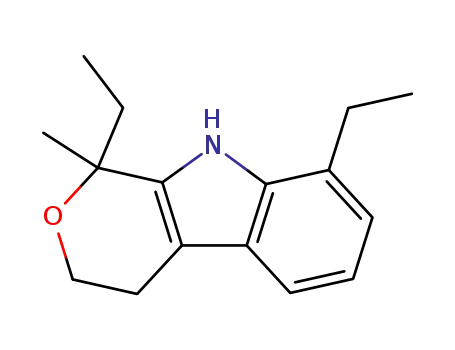
1,8-diethyl-1-methyl-1,3,4,9-tetrahydropyrano-<3,4-b>indole
-
87226-41-3
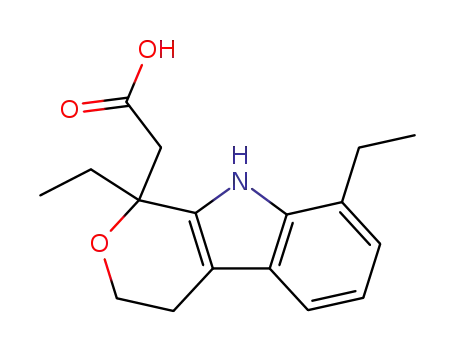
(-)-etodolac
-
111478-86-5
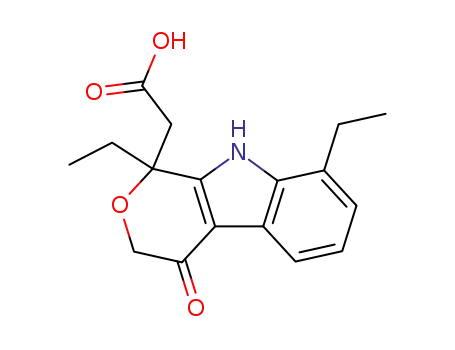
1,8-diethyl-4-oxo-1,3,4,9-tetrahydropyrano[3,4-b]-indole-1-acetic acid
Relevant Products
-
Apixaban
CAS:503612-47-3
-
Neomycin Sulphate
CAS:1405-10-3
-
Denosumab
CAS:615258-40-7