283173-50-2
- Product Name:Rucaparib
- Molecular Formula:C19H18FN3O
- Purity:99%
- Molecular Weight:323.37
Product Details:
CasNo: 283173-50-2
Molecular Formula: C19H18FN3O
Quality Manufacturer Supply Rucaparib,Export 283173-50-2 Efficient Transportation
- Molecular Formula:C19H18FN3O
- Molecular Weight:323.37
- Boiling Point:625.248 °C at 760 mmHg
- PKA:14.10±0.20(Predicted)
- Flash Point:331.938 °C
- PSA:56.92000
- Density:1.281 g/cm3
- LogP:3.69900
Rucaparib (Cas 283173-50-2) Usage
|
Description |
Rucaparib is a first-in-class pharmaceutical drug that targets the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It belongs to the class of PARP inhibitors, which work by blocking the activity of PARP enzymes involved in repairing damaged DNA in cancer cells. By inhibiting PARP, rucaparib prevents cancer cells from repairing DNA damage, leading to their death. |
| Uses |
Rucaparib is approved for various indications related to the treatment of ovarian cancer: Maintenance therapy: Rucaparib is indicated as maintenance treatment for patients with recurrent high-grade ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have responded to platinum-based chemotherapy. It is specifically used in patients with certain types of inherited (germline) or acquired (somatic) abnormal BRCA genes. Treatment of BRCA-mutated ovarian cancer: Rucaparib is approved for the treatment of adult patients with BRCA-mutated ovarian cancer who have received two or more lines of chemotherapy. Maintenance therapy after platinum-based chemotherapy: Rucaparib is also indicated as maintenance therapy for adult patients with recurrent or relapsed ovarian cancer who have achieved complete or partial response to platinum-based chemotherapy. |
283173-50-2 Relevant articles
Stereospecific PARP Trapping by BMN 673 and Comparison with Olaparib and Rucaparib
J Murai,SYN Huang,A Renaud,Y Zhang,J Ji,S Takeda,J Morris,B Teicher,JH Doroshow,Y Pommier
, Molecular Cancer Therapeutics, 2014
Here, we evaluated the novel PARP inhibitor, BMN 673, and compared its effects on PARP1 and PARP2 with two other clinical PARP inhibitors, olaparib and rucaparib, using biochemical and cellular assays in genetically modified chicken DT40 and human cancer cell lines. Although BMN 673, olaparib, and rucaparib are comparable at inhibiting PARP catalytic activity..
Results of ARIEL2: A Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis
Iain A. McNeish,Amit M. Oza,RL Coleman,Clare L. Scott,Elizabeth M. Swisher
, Journal of Clinical Oncology 2015/05/20
Ovarian cancer remains the most lethal gynecologic malignancy. Although standard platinum-based chemotherapy results in high response rates, more than 70% of patients...
Therapeutic Potential of the Poly(ADP-ribose) Polymerase Inhibitor Rucaparib for the Treatment of Sporadic Human Ovarian Cancer
Maike Ihnen; Christine zu Eulenburg; Teodora Kolarova; Jing Wei Qi; Kanthinh Manivong; Meenal Chalukya; Judy Dering; Lee Anderson; Charles Ginther; Alexandra Meuter; Boris Winterhoff; Siân Jones; Victor E. Velculescu; Natarajan Venkatesan; Hong-Mei Rong; Sugandha Dandekar; Nitin Udar; Fritz Jänicke; Gerrit Los; Dennis J. Slamon; Gottfried E. Konecny
CANCER THERAPEUTICS INSIGHTS| JUNE 09 2013
Drug interactions with rucaparib were synergistic for topotecan, synergistic, or additive for carboplatin, doxorubicin or paclitaxel, and additive for gemcitabine. Synergy was most pronounced when rucaparib was combined with topotecan, which resulted in enhanced apoptosis, DNA fragmentation, and γH2AX formation.
283173-50-2 Process route
-
-
C19H16FN3O

-
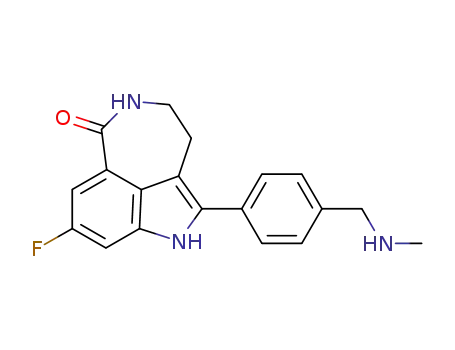
- 283173-50-2
Rucaparib
| Conditions | Yield |
|---|---|
|
C19H16FN3O; With sodium tetrahydroborate; In ethanol; for 1h;
With sodium hydroxide; In water; at 10 ℃; for 2h;
|
90.83% |
|
With palladium 10% on activated carbon; hydrogen; for 12h; Reagent/catalyst;
|
84% |
|
Multi-step reaction with 2 steps
1.1: sodium tetrahydroborate / methanol; tetrahydrofuran / 3 h / 0 - 20 °C / Large scale
1.2: 1 h / Large scale
1.3: 18 h / Large scale
2.1: sodium hydroxide / methanol; water; tetrahydrofuran / 18 h / 20 °C / Large scale
With sodium tetrahydroborate; sodium hydroxide; In tetrahydrofuran; methanol; water;
|
|
|
Multi-step reaction with 2 steps
1.1: sodium tetrahydroborate; methanol / tetrahydrofuran / 2 h / 0 - 20 °C
1.2: 2 h
2.1: sodium hydroxide / water; methanol / 12 h / 0 - 5 °C
With methanol; sodium tetrahydroborate; sodium hydroxide; In tetrahydrofuran; methanol; water;
|
-
![[4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol-2-yl)-benzyl]-methyl-carbamic acid methyl ester](/upload/2024/1/bf01f982-dd1b-4202-92ab-edf170c5870c.png)
- 880160-69-0
[4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol-2-yl)-benzyl]-methyl-carbamic acid methyl ester

-

- 283173-50-2
Rucaparib
| Conditions | Yield |
|---|---|
|
With hydrogen bromide; acetic acid; at 20 ℃; for 12h;
|
89.7% |
|
[4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol-2-yl)-benzyl]-methyl-carbamic acid methyl ester; With hydrogen bromide; acetic acid; at 20 ℃; for 46h;
With sodium hydroxide; In water; at 0 - 35 ℃; pH=10;
|
88% |
283173-50-2 Upstream products
-
880160-69-0
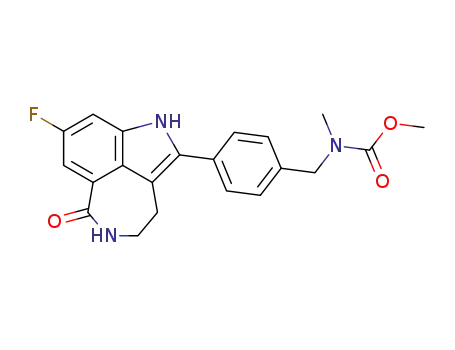
[4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol-2-yl)-benzyl]-methyl-carbamic acid methyl ester
-
283173-84-2
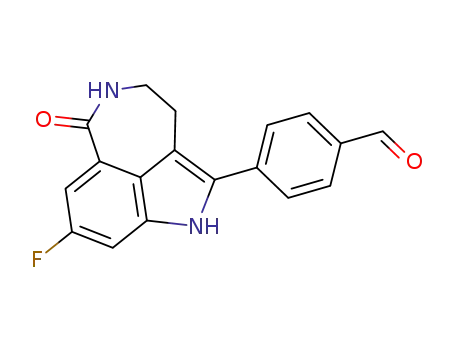
4-(8-fluoro-6-oxo-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd] indol-2-yl)-benzaldehyde
-
283172-68-9
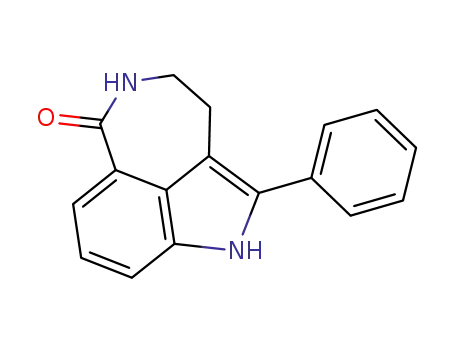
2-phenyl-3,4,5,6-tetrahydro-1H-azepino[5,4,3-cd]indol-6-one
-
773059-19-1
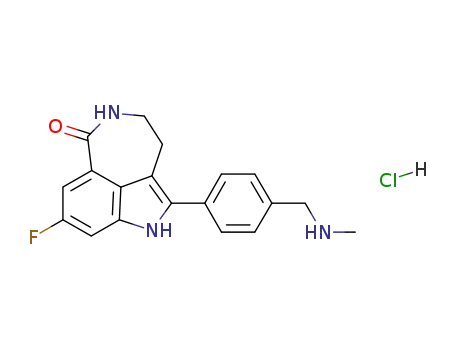
8-fluoro-2-((4-methylaminomethyl)phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one hydrochloride
283173-50-2 Downstream products
-
773059-20-4
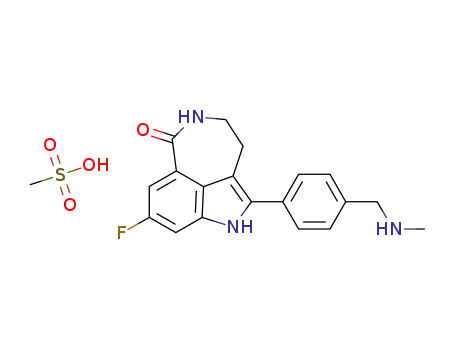
8-fluoro-2-(4-methylaminomethylphenyl)-1,3,4,5-tetrahydroazepino[5,4,3-cd]indol-6-one mesylate
Relevant Products
-
Pralsetinib
CAS:2097132-94-8
-
Abemaciclib
CAS:1231929-97-7